Rev. Mar. y Cost. ISSN 1659-455X. Vol. 1. 9-27, Diciembre 2009.
DOI: http://dx.doi.org/10.15359/revmar.1.1
SPATIAL AND SEASONAL VARIABILITY OF THALASSIA TESTUDINUM IN NUEVITAS BAY, CUBA
Beatriz Martínez-Daranas1*, Rubén Cabrera2 and Fabián Pina-Amargós3
1 Centro de Investigaciones Pesqueras. Ave. 1ra., y 286, Santa Fe, Playa, Ciudad Habana 19100, Cuba. *bmdaranas@yahoo.es
2 Gabinete de Arqueología. Tacón No. 12, Habana Vieja, Cuba. cabrera@arq.patrimonio.ohc.cu
3 Centro de Investigaciones de Ecosistemas Costeros. Cayo Coco, Ciego de Ávila 69400, Cuba. fabian@ciec.fica.inf.cu
Recibido 8-I-2009
Aceptado 16-IV-2009
ABSTRACT
A study was carried out on biomass, shoot density and leaf production variability in three Thalassia testudinum meadows under different environmental characteristics in Nuevitas Bay, Cuba, in different seasons. The first site has muddy-sandy sediments and it is affected by bottom trawl fishing; the second has sandy sediments and it is affected by waste-water discharges, and the third has sandy-muddy sediments and no human impacts are present. Leaf, rhizome, and root biomass, daily production of leaves, density of short shoots, and length and width of leaves were estimated five times in a year. Seasonal variations were observed, with higher values of leaf and rhizome biomass, short shoot density, and daily production of leaves occurring in spring and summer. Spatial differences seem to be related to the environmental characteristics of each site: Leaf daily production, leaf biomass and leaf length were lower, and root biomass was higher in the site where sediments are impacted by fishing with bottom trawls; short shoot density and rhizome biomass were higher in the nonaffected site. Lower values of root biomass appeared where waste-water discharges occurred.
Keywords: Biomass, shoot density, leaf production, seasonal variation, Thalassia testudinum, bottom-trawl fisheries, sewage.
RESUMEN
Se realizó un estudio de la variación de la biomasa, la densidad de vástagos y la producción de hojas en tres praderas de Thalassia testudinum con diferentes características ambientales en la bahía de Nuevitas, Cuba, en diferentes épocas del año. El primer sitio tiene sedimentos fango-arenosos y está afectado por pesquería de arrastre; en el segundo, el sedimento es arenoso y se vierten aguas residuales en su cercanía, y el tercero, con sedimentos arenoso-fangosos, no está afectado por impactos antropogénicos. La biomasa de hojas, rizomas y raíces, la producción diaria de hojas, la densidad de vástagos, y el largo y ancho de las hojas fueron estimadas cinco veces en un año. Se observaron variaciones estacionales, con los valores más altos de biomasa de hojas y de rizomas, densidad de vástagos y de la producción diaria de hojas en primavera y verano. Las variaciones espaciales parecen estar relacionadas con las características de cada sitio: La producción diaria de hojas, la biomasa de hojas y su longitud fueron menores, y la biomasa de raíces mayor, donde los sedimentos están impactados por la pesquería con arrastre; la densidad de vástagos y la biomasa de rizomas fueron mayores en el sitio no afectado por ninguna acción antrópica. Los menores valores de la biomasa de raíces aparecieron donde se realiza la descarga de residuales.
Palabras claves: Biomasa, densidad de vástagos, producción foliar, variaciones estacionales, Thalassia testudinum, pesquería de arrastre, aguas residuales.
INTRODUCTION
Seagrass meadows world-wide are composed by a small number of marine angiosperm species. They are highly productive ecosystems, comparable to most efficient ecosystems on Earth (Duarte & Chiscano, 1999). They are clonal plants that colonize space successfully through the replication of ramets as a result of extension of the rhizome (Duarte et al. 2006). Plasticity in the growth-form of seagrasses is an important component of their capacity to adapt to the heterogeneity of resources and is a result of physiological changes in response to environmental conditions. Therefore, certain seagrass characteristics such as biomass and shoot density can be used as an indicator of diverse impacts on their growth (Kirkman, 1996).
Moreover, water-column nutrient concentrations themselves tend to be poor indicators of nutrient status because water column nutrients (phosphorus and nitrogen) can have turnover rates of less than 10 min. For that reason, traditional water-quality monitoring programs can fail to detect nutrient induced declines in seagrass health, and seagrass meadows should be monitored directly to determine status and/or trends in the health of estuarine environments (Tomasko et al. 1996). For using seagrass characteristics as indicators in monitoring, it is necessary to understand their variability.
Seasonal variability of abundance, productivity and morphometric characteristics in seagrass meadows has been documented in temperate meadows (i.e. Gobert et al. 2006; Moore & Short, 2006). Although tropical habitats seems to be more stable than temperate ones concerning to temperature and light regimes, tropical seagrasses also experience differences according to season (Gacia, 1999; Martínez-Daranas et al. 2005a; van Tussenbroek et al. 2006).
Many authors have documented the disturbance through human activity in seagrass habitats as rates of declining are about 1 to 2% yr-1 on a global scale for several decades, and these rates seems to be accelerating during the last years placing them among the most vulnerable ecosystems in the planet (Fourqurean & Robblee, 1999; Orth et al. 2006). This decline has multiple causes where eutrophication (Short & Wyllie-Echeverría, 1996; Borum et al. 2004; Orth et al. 2006; Ralph et al. 2006) and physical actions due to human activities, as dredging, boating and bottom trawl fisheries, are among the most impacting factors worldwide (Leriche et al. 2006; Erftmeijer & Lewis 2006; Gillanders 2006; Badalamenti 2006).
Thrush & Dayton (2002) summarizes the deleterious effects of physical actions as trawling and dredging on marine benthic habitats as impacts on community structure and physical changes in habitat structure, along with functional changes to seafloor ecosystems. Among the long-term physical effects of bottom fishing are: the homogenization of the substratum that will affect the survivorship of juvenile life stages of species (including some of the target species of fisheries); consequently, the reduction of species diversity; the disturbance of the seafloor, enhancing the upward flux of nutrients by releasing pore-water nutrients with potentially ecosystem-wide consequences on phytoplankton growth. All these changes lead to changes in the functional roles played by organisms, communities, and ecosystems, making unable to fulfil their natural roles in community and ecosystem function.
In Nuevitas Bay, as in many coastal zones around the world, different socio-economic uses of the coastal zone are overlapped, such as: industry wastes, port, fishing, tourism and recreational activities. This bay is surrounded by a border of nearly 2 000 m of Rhizophora mangle, which is included in the protected area “Ballenatos Keys and Nuevitas Bay Mangrove Swamp Fauna Refuge,” where the manatee Trichechus manatus and the crocodile Crocodilus acutus have their habitat (Alcolado et al. 1999). In the other hand, this area also receives 1 128 t of organic load in DBOd terms, as well as waste waters enriched with heavy metals and inorganic nitrogen from several industries (power plant, factories of fertilizer, cement and wires), port activities and direct un-treated waste waters from a city with more than 46 000 inhabitants (Montalvo et al. 2007). An early study on seagrasses from Nuevitas Bay revealed a relationship between loss of dry biomass and species richness decline, with seawater turbidity in areas of this bay (Martínez-Daranas et al. 1996).
The objective of this work was to study the possible seasonal variability of some characteristics of Thalassia testudinum Banks ex König in three areas of Nuevitas Bay, and to evaluate the impact of different anthropogenic factors such as bottom-trawl fisheries and sewage, on these seagrass meadows.
MATERIALS AND METHODS
Study site
Nuevitas Bay is located in the North-eastern Cuban shelf and it is subdivided into two lobes (Figure 1): a shallow Northern lobe called Mayanabo Inlet, where seagrasses are poorly developed, and the Southern lobe (properly Nuevitas Bay), where T. testudinum meadows cover the bottom. Other species of seagrasses (Syringodium filiforme, Halodule wrightii, Halophila decipiens and Halophila engelmanni) and macroalgae (mainly Bryopsidales, Chlorophyta) are frequently found in this bay (Martínez-Daranas et al, 1996). Commercial fishing with bottom trawls and the spill of domestic and industrial wastes from Nuevitas city are threats to marine ecosystems in this bay.
Three sites were selected (Figure 1) to study the spatial and seasonal variability in the following parameters: biomass, density, leaf length and production of seagrass T. testudinum in monospecific stands 2 m deep. The first site (identified as sewage site or SS) is near to industrial (wire and electrode factory) and urban sewage drains, with a muddy-sandy substrate. The second site (identified as trawling site or TS) is located about 6 km South-southeast of site 1, and it is exposed to trawling fisheries, with sandy sediments. The third site (considered as control site or CS) is far away from the disturbance sources, near the bay´s entrance channel, with constant exchange with clean seawater, and sandy-muddy sediments.
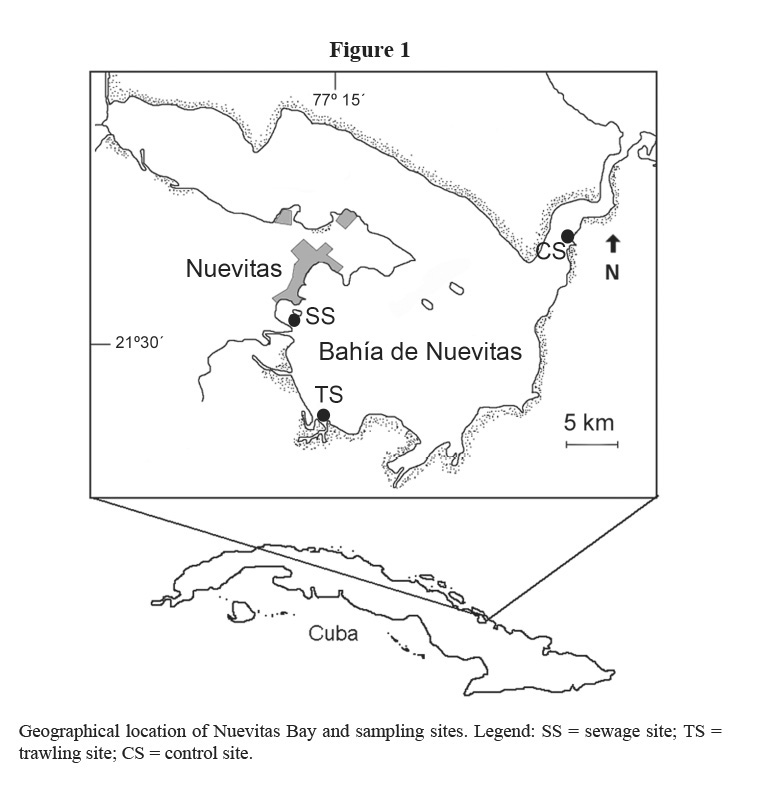
Nuevitas Bay seawater has been classified in general as mesotrophic to eutrophic, according to nutrients and phytoplankton (Montalvo et al. 2007). Based on different studies undertaken from 1994 to 2005, these authors considered that in the area where the sewage site (SS) is located, the quality of water and sediments is worse than in the trawling site (TS). The best water quality was found around the control site (CS).
Sampling and analysis
Samplings were done in February, August and October 2001, and January and April 2002 at each site. The above- and belowground plant sections were harvested using a PVC core of 15 cm of internal diameter that was introduced up to approximately 15 cm in the sediment, using four sampling units each time, following the methodology of CARICOMP (2001). Sediment was removed with running water and calcareous epiphytes were eliminated with 5% HCl. Collected material was separated in leaves, vertical and horizontal rhizomes, and roots. Each tissue group was placed in aluminium foils, previously weighed, and dried at 70oC for 24 h., until a constant weight was reached.
The daily production of leaves was estimated using the Zieman (1975) method, marking the leaves with a hypodermic needle and using twelve sampling units with 10 x 25 cm quadrates, leaving them for 8-12 days before collecting the leaves. At this time, the density of short shoots was estimated.
Fifteen short shoots were randomly taken in each site and the most developed and complete leaf of each shoot was used to calculate the length of leaves with a ruler. The width of leaves was estimated using a binocular microscope.
Data normality and homogeneity of variance were tested (Zar, 1996) and the variables that did not match these assumptions were log transformed. A Two-Factor ANOVA was applied to the measured variables and the Newman-Keuls (SNK) post-hoc comparison test was performed when significant differences existed. All the statistical tests and graphs were carried out using Statistica 6.0 (© Statsoft Inc., 1984-1995) with a 5% probability.
RESULTS
Seasonal trends were similar for the three sampling sites with respect to leaf, rhizomes and root biomass, as well as for short shoot density and leaf length for T. testudinum, as the F-value for the interaction of these variables were not significant (Table 1).
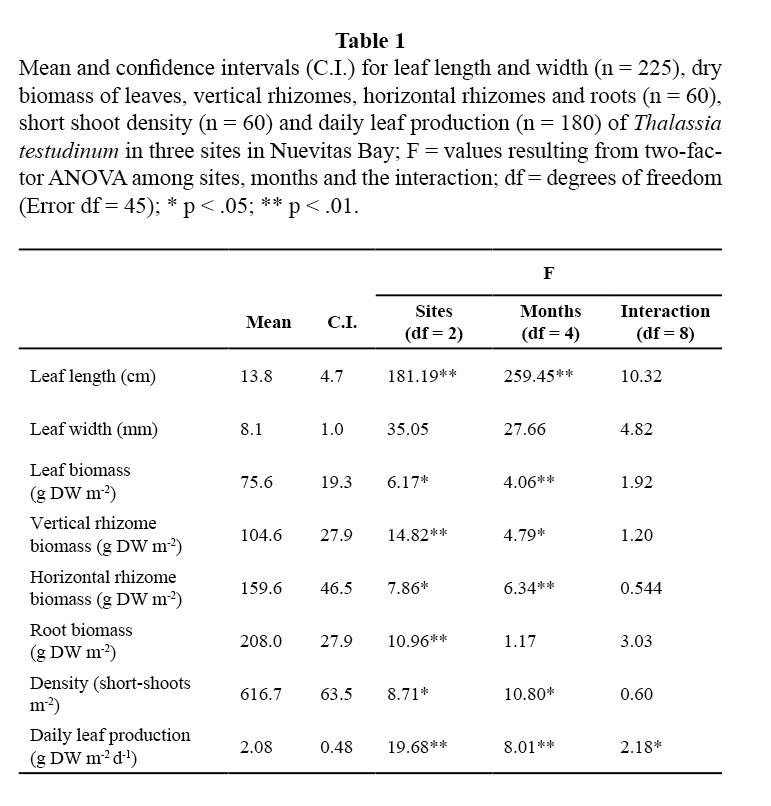
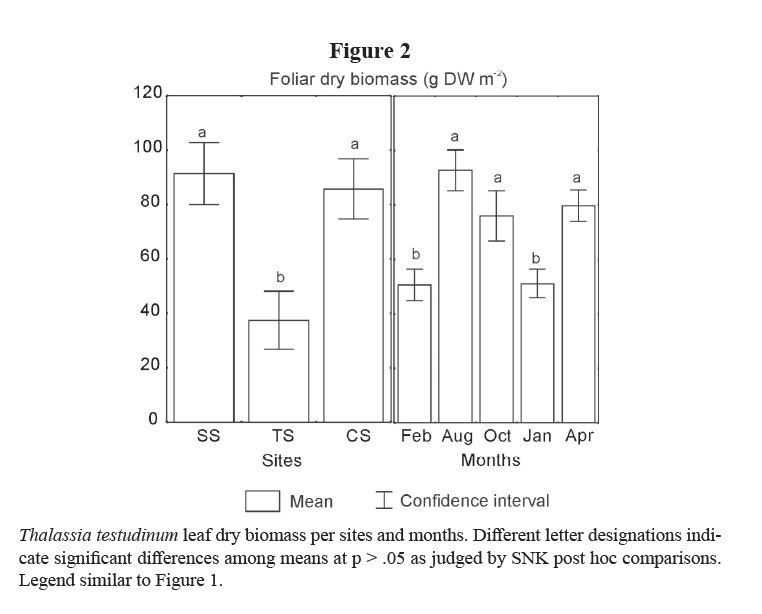
Leaf biomass of this species showed lower means in TS and in the winter months (February 2001 and January 2002; Table 1; Figure 2). Vertical rhizomes biomass was lower in TS and higher in CS, and it was lower in winter months than in the others (Table 1; Figure 3). Horizontal rhizomes showed the same spatial and seasonal differences as the vertical ones. Root biomass showed significant differences only among sites, lower in SS and higher in TS (Table 1; Figure 4).

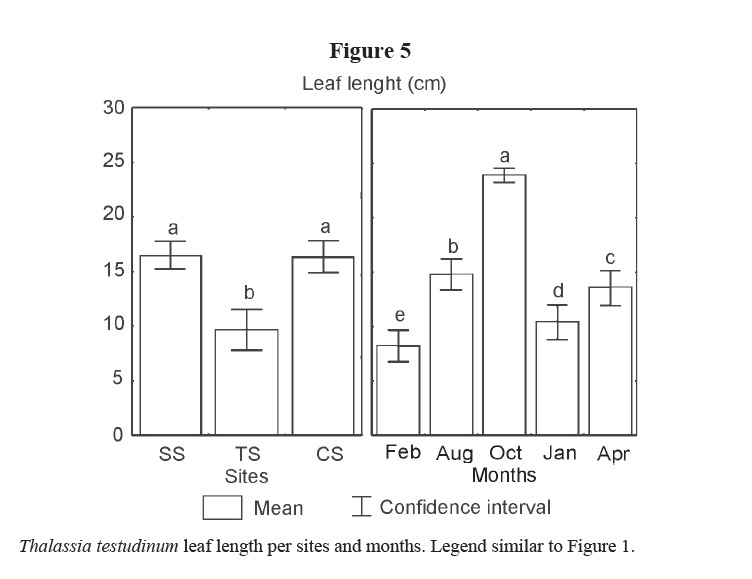
Leaf width did not show differences (Table 1). Lower values of leaf length were found at TS, and among months, it was lower in winter, while in October 2001 it presented the highest value (Figure 5).
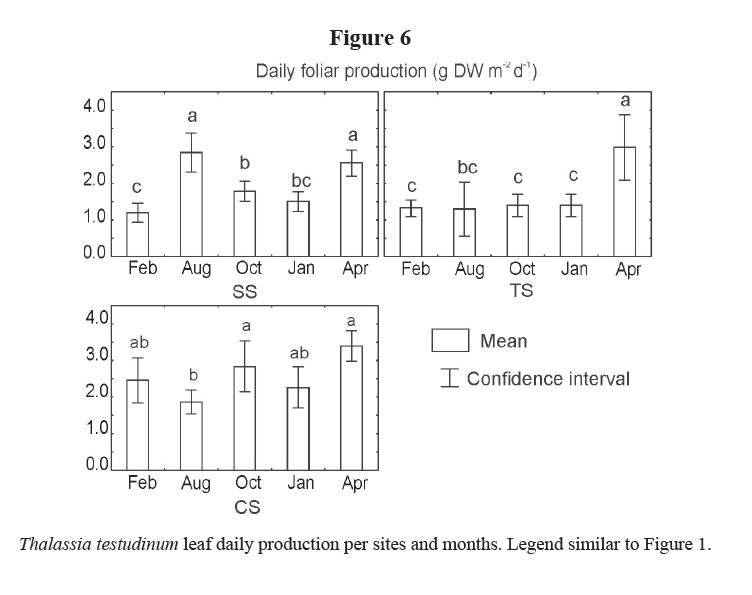
Daily production of leaves showed different trends among the three sites, months, and the interaction of the two factors (Table 1). Higher values were generally found in CS, although they were higher in April 2002 for all sites, as well as in August 2001 in SS (Figure 6). Lower production was detected in February 2001 in SS, August 2001 in CS and during winter months in TS.
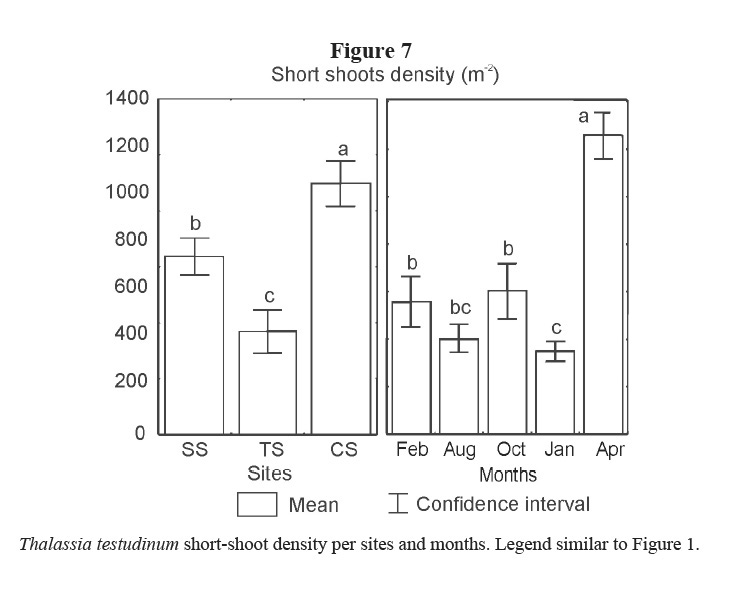
Short shoot density was higher in CS and lower in TS. Minimum density was found in January 2002 and maximum in April 2002 for all sites (Table 1, Figure 7).
DISCUSSION
Temporal variations
Seasonal variations in tropical areas are weaker than in temperate regions; but fluctuations in temperature, radiation, rain, and storms can explain seasonal changes in production in the tropics (Hemminga & Duarte, 2000; Zieman, 1975). In the North coast of Cuba, higher values of T. testudinum leaf biomass and short shoot density were found in spring (March-April; Buesa, 1974; Jiménez & Alcolado, 1989; Martínez-Daranas et al. 2005a), and higher leaf production in summer (June; Martínez-Daranas et al. 2005a). In the Indian River, Florida, which is located at a higher latitude than Cuba, T. testudinum leaf biomass and short shoot density were higher in mid-summer (July) and lower by the end of the winter (Gacia, 1999).
In Nuevitas bay, T. testudinum leaf and rhizome biomass as well as leaf length presented lower values in colder months (February 2001 and January 2002). As a result, total biomass of T. testudinum was lower in winter. The whole growth of the plant and the accumulation of reserves in the rhizomes depend on the photosynthetic activity in the leaves (Hemminga & Duarte, 2000; Marbá & Duarte, 1998). Therefore any factor that causes a reduction of photosynthesis will have a negative incidence on non-photosynthetic biomass (root and rhizomes) biomass.
Variations in leaf biomass are the result of the leaf area, the short shoot density and the number of leaves. In this study, leaf width did not show differences. Leaves were shorter in winter probably due to the effects of cold fronts that caused the detachment and breaking of leaves, and the lower production of tissues induced by low temperatures. The increment in length from August to October can be the result of aging leaves. This result coincides with Agawin et al (2001) for T. hemprichii in the Philippines, and with Durako & Moffler (1985) for T. testudinum in Tampa Bay. The density of short shoots showed highest values in spring (April 2002), and the lowest in January 2002; but not so low in February 2001. Thus, the highest values of leaf biomass must be mainly the result of leaf length in October 2001 and of density of short shoots in April 2002.
Similar seasonal patterns for above-ground blade dynamics, blade length, and number of blades per shoot, as well as for the contribution of T. testudinum blades to total plant biomass have been found in Puerto Morelos, Mexico, with higher values in summer (van Tussenbroek, 1995, 1998). This author did not find variations for rhizome weight per unit length between summer and winter, what might indicate that this seagrass bed has a positive carbon balance throughout the year (van Tussenbroek, 1998).
Changes in rhizome biomass observed in this work can be due to variations in the storage of carbohydrate reserves (Larkum et al 2006). The storage capacity of horizontal rhizomes is usually higher than that of the vertical ones, but both change during the seasons (Hemminga & Duarte, 2000). In the Gulf of Mexico, Lee & Dunton (1997) found that non-structural carbohydrates of T. testudinum in Laguna Madre (Texas, USA) increased in summer, and that this species required an annual quantum flux in excess of 1628 mol m−2 in order to maintain a positive carbon balance, which corresponded with 14% of the surface irradiance. Tomasko & Dawes (1989) experienced with T. testudinum shaded shoots and concluded also that the light environment is an important factor in the carbon balance and in the translocation of resources from rhizomes to leaves and meristematic tissues.
The daily production also shows temporal variations and differences among sites. Daily leaf production increased in April 2002 for the three studied sites, similar to other zones in Cuba (Martínez-Daranas et al. 2005a). Lower values were found mostly in winter for SS and TS; but lower values were also found in August 2001 for CS and in October 2001 for SS. These differences cannot be explained at this time and may be due to other factors not analyzed in this study.
Seasonal differences in several characteristics of T. testudinum as leaf production, biomass or short shoot density have been found in several studies (i.e. Buesa, 1974; Durako & Moffler, 1985; Jiménez & Alcolado, 1989; van Tussenbroek, 1995, 1998; ; ; this study). Therefore, seasonal variability must be considered for detecting reliable differences among locations or dates in monitoring seagrass health.
Spatial variations
Leaf and rhizome biomass, leaf length and density of short shoots were lower in the site affected by bottom trawl fisheries (TS). These effects could be due to physical damage on plants and on sediments caused by the use of fishing gears in the area. Similarly, Martínez-Daranas et al (2005b) found lower values of macroalgal relative abundance and species heterogeneity (H´) in TS, when comparing with the other two sites. Root biomass was higher in TS site as well as the proportion of root-biomass: total-biomass when it is compared with the two other sites (Figure 8). A higher development of roots could help the plant get nutrients from the pore water and fasten better to the sand, coinciding with the results of Dawes & Lawrence (1979) and Kimbel & Carpenter (1981). Bottom trawl fisheries stopped in February 2002 and two months later, in April 2002, leaf production increased significantly, thus supporting the idea that these actions affected T. testudinum meadow at this site, and strengthening the criteria that T. testudinum has a high tolerance to burial and erosion of sediments (Cabaço et al. 2008). Those results coincide with González-Correa et al (2005) who assessed the recovery capacity of meadows of the Mediterranean seagrass Posidonia oceanica in an area affected by illegal trawling and they found that, as in this case, the vegetative growth was positive in both impacted and control meadows, but growth rates were lower in the bottom trawl fisheries site than in control one. This sort of fishery explains the lower values of rhizome biomass due to photosynthetic tissue loss and sediments resuspension, reducing light availability (Pascualini et al. 1999; González-Correa et al. 2005). Fishing can also disturb sediments, causing the loss of nutrients and hydrogen sulphide in the pore water as well as minor particles of sediments, changing their characteristics (Thrush & Dayton, 2002; Gillanders, 2006).
The presence of finer sediments in sites nonaffected by bottom trawling can be explained also because seagrasses usually lead to increased net sedimentation rates and reduction of the grain size (Bos et al. 2007). Higher silt content in dense seagrass beds is the result of waves and currents attenuation and sediments stabilization by seagrass leaves (Koch et al. 2006).
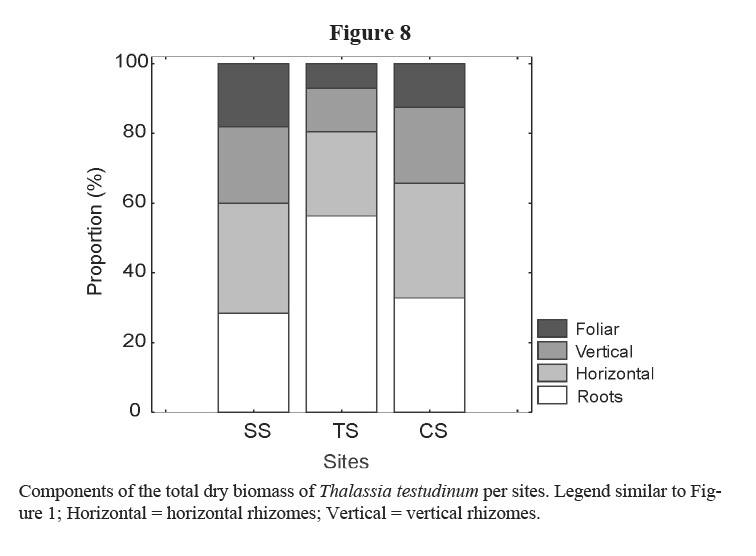
In studied sites, differences were not detected in leaf biomass and leaf length between SS (site affected by waste water) and CS (control site), but rhizome and total biomass, as well as short shoot density were lower in the first than in the latter. Daily leaf production was also generally lower in SS, except in August 2001 and in April 2002. This can be due to the influence of nutrients that could enhance macroalgae development, reducing light availability. Martínez-Daranas et al (2005b) found the highest values of macroalgae abundance and diversity in this site, in the same period, with many opportunistic species such as Cyanobacteria, filamentous (Ceramiaceae, Ectocarpales and Sphacelariales), foliose (Ulvaceae, Dictyotaceae) and fleshy macroalgae (Gracilariaceae) growing in this seagrass meadow and as epiphytes.
Many authors state that, as nutrient inputs raise, micro and macroalgae, and phytoplankton biomass in the water column increase, reducing the light available for photosynthesis which may result in a negative carbon balance (Hemminga & Duarte, 2000; Lapointe, 1989; Tomasko & Lapointe, 1991). The capacity to store carbohydrate reserves in the rhizomes allows seagrasses to survive unfavourable periods, such as shading associated with sporadic algal blooms; but these reserves cannot prevent mortality when light reduction is imposed over long time spans. Light reduction is the most important factor responsible for seagrass decline in eutrophic waters, because sediments can become anoxic; therefore, meristematic tissues of Thalassia can be affected if eutrophication lasts for long periods. The internal transport of oxygen in seagrasses is predominantly unidirectional from photosynthetic tissues to rhizomes to roots driven, and insufficient oxygen supply to seagrass meristems and roots may have severe implications for seagrass growth and survival. Tissue anoxia impairs growth of roots, nutrient uptake and translocation of nutrients and carbohydrates, and the disappearance of the oxic microshield around roots and rhizomes, normally provided by the radial oxygen loss, allows the invasion of reduced phytotoxins, such as sulphides, from the sediment to the plant tissues (Borum et al. 2006).
Unpublished data obtained in the study area in April 2002 by the Laboratory of Chemistry at the Instituto de Oceanología (José F. Montalvo, personal communication) showed that in SS the values of BOD5 and ammonium in water, as well as the content of organic carbon, total phosphorus and hydrogen sulphide in sediments, were higher than in the other two sites (Table 2). Lower values of root biomass were found in SS, the site impacted by waste waters, where the sediment is muddy and total phosphorus concentration in sediment was higher than in the other two sites (Table 2).

CS presented the highest mean values of rhizomes and total biomass, short shoot density and daily leaf production. This site is more remote and pristine, far away from pollution sources and fishing gears are not used. The abundance and diversity of macroalgae in this site was between SS and TS and mainly dominated by calcareous species, such as Bryopsidales (Halimeda, Udotea, Caulerpa and Penicillus) and abundant coralline red algae (Martínez-Daranas et al. 2005b). According to Lapointe et al (1994) and Littler & Littler (2007), calcareous species have slow growth rates and are characteristic of areas with low level of nutrients, indicating a stable and constant condition.
Conclusions
Variations found in the studied parameters for T. testudinum in Nuevitas Bay are the outcome of morphologic and physiologic responses to natural environmental conditions. Seasonal changes in light and temperature explain its seasonal differences in biomass, density and productivity. Physical impacts as light reduction induced by eutrophication, or direct physical damage by fishing gears, affect the foliar production, biomass and short shoots density of T. testudinum. According to these results, monitoring biomass and density of seagrasses in Nuevitas bay will be helpful to monitor effects of anthropogenic actions, considering seasonal variations.
ACKNOWLEDGMENTS
This paper is part of the thesis to obtain the Master in Sciences title of the second author at the University of Habana. We thank Juan Manuel López-Bautista, Michael Marshall and Ligia Collado for their suggestions, and José F. Montalvo for providing information about water quality. We are also grateful the anonymous reviewer for their comments which greatly improved the manuscript.
REFERENCES
Agawin, N. R. S., Duarte, C. M., Fortes, M. D., Uri, J. S. & Vermaat, J. E. (2001). Temporal changes in the abundance, leaf growth and photosynthesis of three co-occurring Philippine seagrasses. J. Exp. Marine Biol Ecol., 260, 217-239.
Alcolado, P. M., García, E. E. & Espinosa, N. (1999). Protección de la biodiversidad y desarrollo sostenible en el ecosistema Sabana-Camagüey. Madrid: CESYTA S. L.
Badalamenti, F., Di Carlo, G., D’Anna, G., Gristina, M. & Toccaceli, M. (2006). Effects of dredging activities on population dynamics of Posidonia oceanica (L.) Delile in the Mediterranean Sea: the case study of Capo Feto (SW Sicily, Italy). Hydrobiol., 555, 253-261.
Borum, J., Duarte, C. M., Krause-Jensen, D. & Greve, T. M. (2004). European seagrasses: an introduction to monitoring and management. The M&MS Project, Copenhagen, Consulted on May 15th /2008 from http://www.seagrasses.org/.
Borum, J., Sand-Jensen, K., Binzer, T., Pedersen, O. & Greve, T. M. (2006). Oxygen movement in seagrasses. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 255-270). Dordrecht, The Netherlands: Springer.
Bos, A. R., Bouma, T. J., de Kort, G. L. J. & van Katwijk, M. M. (2007). Ecosystem engineering by annual intertidal seagrass beds: Sediment accretion and modification. Estuar. Coast. Shelf. Sci., 74, 344-348.
Buesa, R. J. (1974). Population and biological data on turtle grass (Thalassia testudinum König, 1805) on the northwestern Cuban shelf. Aquaculture, 4, 207-226.
Cabaço, S., Santos, R. & Duarte, C. M. (2008). The impact of sediment burial and erosion on seagrasses: A review. Estuar. Coast. Shelf. Sci., 79, 354-366.
CARICOMP (2001). CARICOMP Methods Manual - Level I. CARICOMP Management Center, University of the West Indies, Mona, Kingston, Jamaica and Florida. Institute of Oceanography, University of South Florida, St. Petersburg Florida, U.S.A., Mona, Kingston, Jamaica.
Dawes, C. J. & Lawrence, J. (1979). Effects of blade removal on the proximate composition of the rhizome of the seagrass Thalassia testudinum Banks ex König. Aquatic Bot., 6, 79-86.
Duarte, C. M & Chiscano, C. L. (1999). Seagrass biomass and production: a reassessment. Aquatic Boty., 65, 159-74.
Duarte, C. M., Fourqurean, J. W., Krause-Jensen, D. & Olesen, B. (2006). Dynamics of seagrass stability and change. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 271-294). Dordrecht, The Netherlands; Springer.
Durako, M. J. & Moffler, M. (1985). Spatial influences on temporal variations in leaf growth and chemical composition of Thalassia testudinum Banks ex König in Tampa Bay. Fla. Gulf Res. Rep., 8, 43-49.
Erftemeijer, P. L. A. & Lewis, R. R. (2006). Environmental impacts of dredging on seagrasses: A review. Mar. Pollut. Bull., 52, 1553-1572.
Fourqurean J. W. & Robblee, M. B. (1999) Florida Bay: History of Recent Ecological changes. Estuar., 22, 345-357.
Gacia, E. (1999). Leaf dynamics and shoot production of the seagrass Thalassia testudinum in the Indian River Lagoon (Florida). Bot. Mari., 42, 97-102.
Gillanders, B. M. (2006). Seagrasses, fish and fisheries. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 503-536). Dordrecht, The Netherlands; Springer.
Gobert, S., Cambridge, M. L., Velimirov, B., Pergent, G., Lepoint, G., Bouquegneau, J. M., Dauby, P., Pergent-Martini, C. & Walker, D. I. (2006). Biology of Posidonia. In Larkum, A.W.D., Orth, R.J. & Duarte, C.M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 387-408). Dordrecht, The Netherlands; Springer.
González-Correa, J. M., Bayle, J. T., Sánchez-Lizaso, J. L., Valle, C., Sánchez-Jerez, P. & Ruiz, J. M. (2005). Recovery of deep Posidonia oceanica meadows degraded by trawling. J. Exp. Mar.Biol. Ecol., 320, 65-76.
Hemminga, M. A. & Duarte, C. M. (2000). Seagrass Ecology. Cambridge: Cambridge University Press.
Jiménez, C. & Alcolado, P. M. (1989). Comportamiento estacional de la biomasa vegetal en un seibadal en Cuba. Bot. Cubana, 71, 1-10.
Kimbel, J. C. & Carpenter, S. R. (1981). Effects of mechanical harvesting on Thalassia testudinum Banks ex König regrowth and carbohydrate allocation to roots and shoots. Aquatic. Bot., 11, 121-127.
Kirkman, H. (1996). Baseline and Monitoring Methods for Seagrass Meadows. J. Environ. Manage., 47, 191-201.
Koch, E. W., Ackerman, J. D., Verduin, J. & Keulen, M. V. (2006). Fluid dynamics in seagrass ecology - From molecules to ecosystems. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 193-225). Dordrecht, The Netherlands; Springer.
Lapointe, B. E. (1989). Macroalgal production and nutrient relations in oligotrophic areas of Florida Bay. Bull. Mar. Sci., 44, 312-323.
Lapointe, B. E., Tomasko, D. A. & Matzie, W. R. (1994). Eutrophication and trophic state classification of seagrass communities in the Florida Keys. Bull. Mar. Sci., 54, 696-717.
Larkum, A. W. D., Drew, E. A. & Ralph, P. J. (2006). Photosynthesis and metabolism in seagrasses at the cellular level. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation (pp. 323-345). Dordrecht, The Netherlands; Springer.
Lee, K. S. & Dunton, K. H. (1997). Effects of in situ light reduction on the maintenance, growth and partitioning of carbon resources in Thalassia testudinum Banks ex König. J. Exp. Mar. Biol. Ecol., 210, 53-73.
Leriche, A., Pasqualini, V., Boudouresque, C.-F., Bernard, G., Bonhomme, P., Clabaut, P. & Denis, J. (2006) Spatial, temporal and structural variations of a Posidonia oceanica seagrass meadow facing human activities. Aquatic Bot., 84, 287–293
Littler, M. M. & Littler, D. S. (2007). Assessment of coral reefs using herbivory/nutrient assays and indicator groups of benthic primary producers: a critical synthesis, proposed protocols, and critique of management strategies. Aquatic Conserv. Mar. Fresh. Ecosystems., 17, 195-215.
Marbá, N. & Duarte, C. M. (1998). Rhizome elongation and seagrass clonal growth. Mar. Ecol. Prog. Ser., 174, 269-80.
Martínez-Daranas, B., Alcolado, P. M. & Duarte, C. M. (2005a). Leaf production and shoot dynamics of Thalassia testudinum by a direct census method. Aquatic Bot., 81, 213-224.
Martínez-Daranas, B., Cabrera, R. & Pina, F. (2005b). Variaciones espacio-temporales de las macroalgas asociadas y epífitas de Thalassia testudinum Banks ex König, en la Bahía de Nuevitas, Cuba. In Memorias Ficología/2005, VII Congreso de Ficología de Latinoamérica y el Caribe, La Habana, Cuba. CD-ROM, ISBN: 959-237-142-3, No. 12, 12 pp.
Martínez-Daranas, B., Jiménez, C. & Alcolado, P. M. (1996). Prospección del macrofitobentos de los fondos blandos del archipiélago Sabana-Camagüey, Cuba. Avicennia, 4/5, 77-88.
Montalvo, J. F., Perigó-Arnaud, E. & Martínez-Canals, M. (2007). La contaminación marina. In Alcolado, P. M., García, E. E. & Arellano, M. (eds.), El ecosistema Sabana-Camagüey, Cuba: Estado actual, avances y desafíos en la protección y uso sostenible de la biodiversidad (pp. 79-83). La Habana; Editorial Academia.
Moore, K. A. & Short, F. T. (2006). Zostera: Biology, ecology and management. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.) Seagrasses: Biology, Ecology and Conservation (pp. 361-386). Dordrecht, The Netherlands; Springer.
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Kenneth, K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Olyarnik, S., Short, F. T., Waycott, M. & Williams, S. L. (2006). A Global Crisis for Seagrass Ecosystems. BioScience, 56, 987-996.
Pascualini, V., Pergent-Martini, C. & Pergent, G. (1999). Environmental impact identification along the Corsican Coast (Mediterranean Sea) using image processing. Aquatic Bot., 65, 311-320.
Ralph, P. J., Tomasko, D. A., Moore, K., Seddon, S. & Macinnis-Ng, C. M. O. (2006). Human impacts on seagrass: Eutrophication, sedimentation and contamination. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation, (pp. 567-593). Dordrecht, The Netherlands: Springer.
Short, F. & Wyllie-Echeverría, S. (1996). Natural and human-induced disturbance of seagrasses. Environ. Conserv., 23, 17-27.
Thrush, S. F. & Dayton, P. K. (2002) Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu. Rev. Ecol. Syst., 33, 449-473.
Tomasko, D. A. & Dawes, C. J. (1989). Evidence for physiological integration between shaded and unshaded short shoots of Thalassia testudinum. Mar. Ecol. Prog. Ser., 54, 299-305.
Tomasko, D. A. & Lapointe, B. E. (1991). Productivity and biomass of Thalassia testudinum as related to water column nutrient availability and epiphyte levels: field observations and experimental studies. Mar. Ecol. Prog. Ser., 75, 9-17.
Tomasko, D. A., Dawes, C. J. & Hall, M. O. (1996). The effects of anthropogenic nutrient enrichment on turtle grass (Thalassia testudinum) in Sarasota Bay, Florida. Estuar., 19, 448-456.
van Tussenbroek, B. I. (1995). Thalassia testudinum leaf dynamics in a Mexican Caribbean reef lagoon. Mar. Biol., 122, 33-40.
van Tussenbroek, B. I. (1998). Above- and below-ground biomass and production by Thalassia testudinum in a tropical reef lagoon. Aquatic Bot., 61, 69-82.
van Tussenbroek, B. I., Vonk, J. A., Stapel, J., Erftemeijer, P. L. A., Middelburg, J. J. & Zieman, J. C. (2006). The biology of Thalassia: Paradigms and recent advances in research. In Larkum, A. W. D., Orth, R. J. & Duarte, C. M. (eds.), Seagrasses: Biology, Ecology and Conservation, (pp 409-439). Dordrecht, The Netherlands; Springer.
Zar, J. H. (1996). Biostatistical analysis. New Jersey: Prentice Hall Inc.
Zieman, J. C. (1975). Seasonal variation of turtle grass, Thalassia testudinum König, with reference to temperature and salinity effects. Aquatic Bot., 2, 107-123.

Revista Ciencias Marinas y Costeras está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.