Rev. Mar. Cost. ISSN 1659-455X. Vol. 3: 43-49, Diciembre 2011.
DOI: https://doi.org/10.15359/revmar.3.3
GROWTH AND SURVIVAL OF THE FRESHWATER MUSSEL, ANODONTITES TRAPESIALIS (LAMARCK 1819), IN A FLOW-THROUGH SYSTEM FOR LONG-TERM HOLDING
EL CRECIMIENTO Y LA SUPERVIVENCIA DEL MEJILLÓN DE AGUA DULCE, ANODONTITES TRAPESIALIS (LAMARCK 1819), EN UN SISTEMA DE FLUJO CONTINUO A LARGO PLAZO
Ricardo C. Lima1*, Angela T. Paes2 and Wagner E. P. Avelar1
1 Laboratory of Malacology, Depto. Biology, FFCLRP, University of São Paulo. Av. Bandeirantes, 3900, Ribeirão Preto, SP, Brazil, 14040-901.
2 UNIFESP, Applied Statistic Department. *occunhalima@gmail.com
Recibido 28-X-2010
Aceptado 15-IV-2011
ABSTRACT
As with other freshwater mussels, Anodontites trapesialis is an endangered and threatened species. Artificial culture has been strongly recommended in recovery plans as a strategy to bolster declining populations, as well as the reintroduction of species to sites within their historic ranges. Our project compares two methods of adult animal management: buried and suspended, focusing on growth and survival of A. trapesialis in a captive environment. Animals were fed with Chlamydomonas spp. After 120 days, weight (soft and hard body) increased by 2.1% in the suspended group and decreased by 1.4% in the buried group. Suspended animals showed higher survival rates than those that were buried. The information provided may be of particular interest to develop future conservation measures for this and other similar endangered species.
Keywords: Freshwater mussel, Mycetopodidae, aquaculture system, adult management, biodiversity.
RESUMEN
Así como otras especies de almejas de agua dulce, Anodontites trapesialis se encuentra en peligro y amenazada de extinción. El cultivo artificial ha sido muy recomendado en los planes de recuperación como una estrategia para mejorar las cifras de población en declive, así como la reintroducción de especies a sitios dentro de sus rangos históricos. Nuestro estudio compara dos métodos de manejo de animales adultos, enterrados y suspendidos, con un enfoque en el crecimiento y la supervivencia de A. trapesialis en un ambiente de cautiverio. Los animales fueron alimentados con Chlamydomonas spp. Al comparar los porcentajes de peso corporal (concha y tejido blando) después de 120 días, un aumento medio del 2.1% se observó en el grupo suspendido y una pérdida media del 1.4% en el grupo enterrado. Los animales suspendidos tuvieron mejores tasas de supervivencia que aquellos que fueron enterrados. La información proporcionada puede ser de especial interés para el desarrollo de las futuras medidas de conservación para esta y otras especies similares en peligro de extinción.
Palabras claves: Mejillón de agua dulce, Mycetopodidae, sistema de acuicultura, manejo de reproductores, biodiversidad.
INTRODUCTION
The decline of many mussel populations has led to several conservation and management programs aimed at preventing mussel extirpation and extinction. Studies have been conducted to determine the feasibility of relocating risk mussel species into aquaculture and hatchery facilities (Ramírez, 2005), and translocating species to areas within their natural range (Martel et al. 2003). Artificial culture of endangered and threatened mussel species has been strongly recommended in recovery plans as a strategy to enhance declining populations, as well as the reintroduction of species to sites within their historic ranges (O’Beirn et al. 1998).
However, standard protocols for cultivating unionids have not been completely developed, even though many populations of threatened and endangered mussel species would benefit from the release of cultured juveniles. The speed with which culture protocols are developed and employed is crucial for preventing the extinction of rare species (Beck, 2001).
Historically, reports on the aquaculture of freshwater mussels have conveyed little detailed empirical information. The studies done thus far have been focused mainly on larvae and juvenile stage (Buddensiek, 1995; O’beirn et al. 1998; Beck, 2001; Henley, 2002; Araujo et al. 2003; McIvor, 2004), and no study has included broodstock conditioning in captive environment.
Anodontites trapesialis (Lamarck 1819), freshwater mussels belonging to the Mycetopodidae family, are found in all main hydrographical basins of South America, east of the Andes, with the exception of the lower São Francisco River and basins in the extreme south. Typical habitat consists of substrates of varying size from sand or muddy terrain to compact clay. A. trapesialis are found in depths less than 20 m, but are generally collected from depths of 1 to 2 m. Unlike other members of Unionoida, mycetopodidae mussels produce a lasidium rather than a glochidium larvae (Simone, 1994; Callil & Mansur, 2007).
Our project focused on factors influencing the growth and survival of A. trapesialis in a captive environment. To this end, we compared two treatments of broodstock management: buried and suspended.
MATERIALS AND METHODS
Adults of A. trapesialis were collected from the marginal region of the Galo Bravo Reservoir, at Ribeirão Preto, SP, Brazil (21º 07’ 06.9” S, 47º 49’ 32.1” W). Animals were located by probing the bottom of the reservoir with feet and hands. Bivalves were transported alive to the laboratory in insulated boxes with enough local water to cover them.
Two hundred mussels were randomly divided into two treatments (two replicates). A control treatment (N = 50) was kept in tanks (40x60x50 cm), buried by a 15 cm layer of sediment (particle size < 600 µm). The other treatment (N = 50) was suspended in 32x28 cm nylon bags (mesh size of 10x3 mm), with three animals each, at intervals of 15 cm between bags, at 50 cm off the bottom, in the same tanks as buried mussels. All treatment units, consisting of flow-through water systems (mean flow was 10.0 L/min), were located in a room with constant temperature and a 12 hour light-12 hour dark cycle. To promote the suspension of algae cells in the water column, two 5.0 cm air diffusers were used in each treatment during 120 days of controlled culture.
Mussels were fed the unicellular green alga Chlamydomonas spp., at a density of 200 000 cells/ml/day once daily, and water was changed in all treatments on a weekly basis. This microalgae species was chosen due to its availability, easy culture, and fast growth in the laboratory.
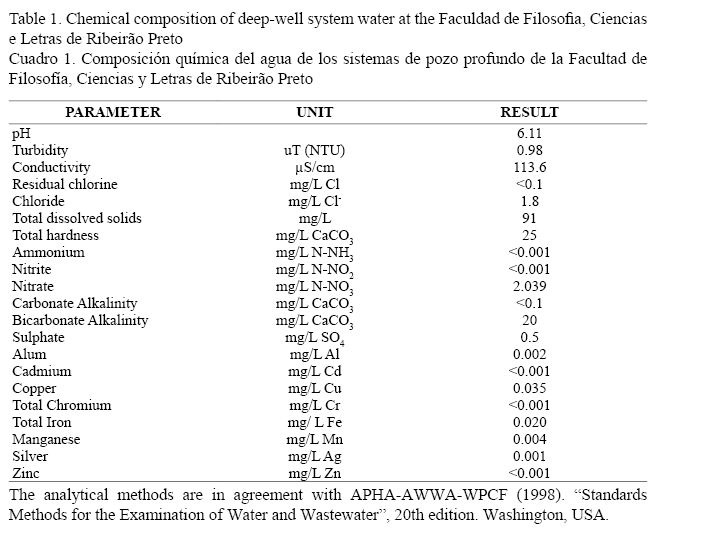
Dechlorinated deep-well system water from the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, São Paulo, Brazil (Table 1) was used for algal cultures and flow-through system. Living algal cultures were maintained in bath cultures in 20 l plastic bottles. Unialgal cultures were not axenic. Enrichment of the algal culture was achieved by the addition of fertilizer (NPK 12:6:6) at a ratio of 1/200.
On days 0, 30, 60, 90 and 120, the wet weight (fresh weight) of each mussel was measured to 0.01 g using a Gehaka BG 400 electronic balance.
Mortality rates of both groups were compared using Pearson´s chi-square test. ANOVA was used with repeated measures considering two factors: treatments (buried and suspended) and time (days 0, 30, 60, 90 and 120). Comparisons between and inter-groups were evaluated by orthogonal contrasts. All tests for significance were done at the 95% confidence level and calculations were performed using SAS software (9.0).
RESULTS
Survival was higher for suspended mussels (96%) than for buried mussels (78%; P = 0.007).
Despite the random assignment of mussels to treatments, initial mean weight of buried mussels was heavier than the initial mean weight of suspended mussels (P = 0.0005).
The ANOVA model demonstrated that the interaction effect between treatment and time was significant (P < 0.001), indicating that the growth patterns of mussels were different between the treatments (Table 2). The suspended group lost weight during the first 30 days (P < 0.001) but later gained it with all differences being statistically significant (P < 0.001). In the buried treatment, there was a significant loss of weight at days 30 and 60 (P < 0.001); however, no differences were observed between days 60 and 90 (P = 0.1226) and between days 90 and 120 (P = 0.6963). Over the 120 day experiment, the mean weight of the buried treatment was higher than the suspended treatment (P < 0.01).
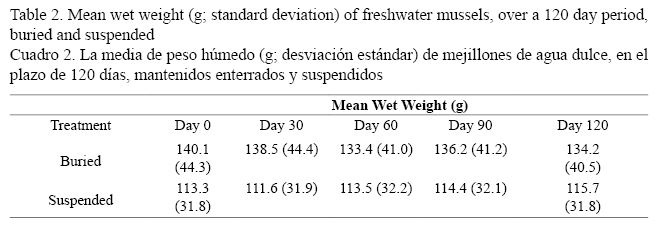
Both treatments lost weight during the first month. However, the suspended mussels gradually regained weight, while the control group initially lost more weight than the suspended mussels and did not regain the initial loss (Table 2). Comparing the experiment over the 120 day, weight of the suspended treatment increased by 2.1% and the buried treatment lost 1.4% of initial mean weight.
DISCUSSION
Most attempts to rear freshwater mussels have focused on North American species, with a few studies on European species (Buddensiek, 1995; Henley, 2002; Araujo et al. 2003; McIvor, 2004). This study represents the first attempt to rear a Brazilian species of freshwater mussel: A. trapesialis, which is of particular importance because this species is currently listed as threatened (Amaral et al. 2008). Its culture may offer both the possibility of reintroducing this species to sites where it has been lost and the opportunity to study its requirements for future laboratory research (McIvor, 2004).
The quality and quantity of suspended food are important to the physiological condition of marine and freshwater bivalves (Henley, 2002), although the diet of adult freshwater mussels is poorly known. It has generally been assumed that adult mussels primarily feed on phytoplankton, the same way as marine bivalves (Gosling, 2003), and most culture studies to date have fed juvenile mussels with a suspension of algae (Hudson & Isom, 1984; Yeager et al. 1994; Gatenby et al. 1997; O’Beirn et al. 1998; Dimock, 2000; Henley et al. 2001; Jones et al. 2005). However, Nichols & Garling (2000) showed that freshwater mussels gained most of their carbon from bacterial sources.
Gatenby et al. (1997) looked at a variety of foods for juveniles, including various algal combinations, bacteria, and sediment. They found that sediment alone supported Villosa iris juveniles, but the addition of algae increased survival and growth. The addition of bacteria to their diet did not increase survival and growth. However, a different study by Yeager et al. (1994) found that three to five-day-old V. iris juveniles contained flagellated bacteria in their guts, with smaller quantities of algal cells. Therefore, it is likely that juveniles gain their nutrition from both small algal cells and bacteria.
The unialgal diet offered, Chlamydomonas spp., supported growth of A. trapesialis, at least for the suspended treatment. Based on the results of this study, the main problem for buried mussels was the high mortality level, which could be due to an anoxic environment within the substrate. It was observed that when a mussel died the substrate close to it turned gray and caused the death of the other neighboring mussels.
The feeding rate for juvenile mussels varies among authors, from 10,000 cells/ml/day (O’Beirn et al. 1998) to 30 000 cells/ml/day (Jones et al. 2005; Henley, 2002). Beck (2001) examined the particle selection by Villosa iris at three different ages and proposed that feeding rations should be increased every 10 days, starting with 30 000 cells/ml/day until reaching the rate of 90 000 cells/ml/day. There is little data on the feeding rate for maintaining captive broodstock freshwater mussels; however, the rate used in this study, 200 000 cells/ml of Chlamydomonas spp., sustained the adults of A. trapesialis, as the animals gained weight.
Martel et al. (2003) working with Elliptio complanata showed that mussels grow better when kept in steel cages rather than individual chambers tied off with a mesh bag, possibly due to their biology. In their natural environment, these mussels are partly imbedded in soft substrate and are mobile. In a steel cage, mussels left unrestricted can orient their position thereby maximizing their ability to obtain nutrients. In contrast, in the study by Martel et al. (2003), the mesh bag supported by the PVC frame may have restricted the ability of E. complanata to orient themselves or open adequately and therefore reduced their ability to feed properly. In the present experiment a nylon mesh bag was used allowing mussels to orient themselves and not disturbing their growth.
Management of suspended mussels is much easier and provides better water conditions based on survival rate. Additionally, microalgae were more available to them due to the use of aeration and because bags were hanging up within the water column. This study demonstrates that growth and survival of A. trapesialis in a captive environment for long-term holding is possible when they are kept suspended, which seems to promote healthier animals for propagation programs. The information provided may be of particular interest for developing future conservation measures for this and other similar endangered species.
It should be noted that there are many possible levels of replication in these experiments, including the different number of juveniles held in each mesh bag or tray, the different number of bags and trays within each tank, and the different sizes of tanks. Where possible in these experiments, replication was at the level of different containers (trays/mesh bags). However, there has been little replication at the level of tanks because it was not known which tanks would support juvenile survival and growth; therefore, it was considered more important to place juveniles in many different tanks, rather than in many tank replicates. This has resulted in some pseudoreplication (as described by Hurlbert, 1984), which is a common problem for aquaculture experiments (Smart et al. 1997, 1998; Riley & Edwards, 1998), and many previous freshwater mussel culture experiments have suffered from the same low levels of replication (e.g. O’Beirn et al. 1998; Jones & Neves, 2002). In future studies, replication at the level of tanks or recirculating systems should be attempted where possible.
ACKNOWLEDGEMENTS
We are very grateful to Bonigo for their donation of fertilizer for the microalgae culture. We would also like to thank Michael Crowe for editing the English, FAPESP for the financial support (grant no. 2006/04658-7), and CAPES for the doctoral fellowship.
REFERENCES
Amaral, A. C. Z., Ribeiro, C. V., Mansur, M. C. D., Santos, S .B., Avelar, W. E. P., Matthews-Cascon, H., Leite, F. P. P., Melo, G. A. S., Coelho, P. A., Buckup, G. B., Buckup, L., Ventura, C. R. R. & Tiago, C. G. (2008). A situação de ameaça dos invertebrados aquáticos no Brasil. In A. B. M. Machado, G. M. Drummond, & A. P. Paglia (Eds.), Livro vermelho da fauna brasileira ameaçada de extinção (pp.157-301). Ministério do Meio Ambiente, Brasília, Brasil.: Série Biodiversidade 19.
Araujo, R., Quirós, S. M., & Ramos, M. A. (2003). Laboratory propagation and culture of juveniles of the endangered freshwater mussel Margaritifera auricularia (Spengler, 1793). J. Conchol., 38(1), 53-60.
Beck, K. M. (2001). Development of an algal diet for rearing juvenile freshwater mussels (Unionidae). Unpublished master´s thesis, Virginia Polytechnic Institute and State University, Blacksburg, Virginia.
Buddensiek, V. (1995). The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: A contribution to conservation programmes and the knowledge of habitat requirements. Biol. Conserv., 74, 33-40.
Callil, C. T. & Mansur, M. C. D. (2007). Gametogênese e dinâmica da reprodução de Anodontites trapesialis (Lamarck 1819) no lago Baía do Poço, planície de inundação do rio Cuiabá, Mato Grasso, Brasil. Rev. Bras. Zool., 24(3), 825-840.
Dimock, R. V. (2000). Oxygen consumption by juvenile Pyganodon cataracta (Bivalvia: Unionidae) in response to declining oxygen tension. In R. A. Tankersley, D. I. Warmolts, G. T. Watters, B. J. Armatage, P. D. Johnson, & R. S. Butler (Ed.), Freshwater Mollusk Symposium Proceedings (pp. 1-8). Ohio. USA.: Ohio Biological Survey, Columbus.
Gatenby, C. M., Parker, B. C., & Neves, R. J. (1997). Growth and survival of juvenile rainbow mussels, Villosa iris (Lea, 1829) (Bivalvia: Uniondoidea), reared on algal diets and sediment. Am. Malacol. Bull., 14, 57-66.
Gosling, E. (2003). Bivalve molluscs: biology, ecology and culture. Malden, USA.: Blackwell Publishing.
Henley, W. F. (2002). Evaluation of diet, gametogenesis and hermaphroditism in freshwater mussels (Bivalvia: Unionidae). Unpublished doctoral dissertation, Virginia Polytechnic Institute and State University, Blacksburg, Virginia.
Henley, W. F., Zimmerman, L. L., Neves, R. J., & Kidd, M. R. (2001). Design and evaluation of recirculating water systems for maintenance and propagation of freshwater mussels. N. Am. J. Aquac., 63, 144-155.Hudson, R. G. & Isom, B. G. (1984). Rearing juveniles of the freshwater mussels (Unionidae) in a laboratory setting. Nautilus, 98(4), 129-135.
Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecol. Monogr., 54(2), 187-211.
Jones, J. W., Mair, R. A., & Neves, R. J. (2005). Factors affecting survival and growth of juvenile freshwater mussels cultured in recirculating aquaculture systems. N. Amer. J. Aquac., 67, 210-220.
Jones, J. W. & Neves, R. J. (2002). Life history and propagation of the endangered fanshell pearlymussel, Cyprogenia stegaria Rafinesque (Bivalvia: Unionidae). J. N. Amer. Benthol. Soc., 21, 76-88.Martel, P., Kovacs, T., Voss, R., & Megraw, S. (2003). Evaluation of caged freshwater mussels as an alternative method for environmental effects monitoring (EEM) studies. Environ. Poll., 124, 471-483.
McIvor, A. L. (2004). Freshwater mussels as biofilters. Unpublished doctoral dissertation, Department of Zoology, University of Cambridge.
Nichols, S. J. & Garling, D. (2000). Food-web dynamics and trophic level interactions in a multispecies community of freshwater unionids. Can. J. Zool., 78, 871-882.
O´Beirn, F. X., Neves, R. J., & Steg, M. B. (1998). Survival and growth of juvenile freshwater mussels (Unionidae) in a recirculating aquaculture system. Am. Malacol. Bull., 14(2), 165-171.
Ramírez, C. A. P. (2005). Evaluación del efecto de la exposición al aire sobre la sobrevivencia de Diplodon chilensis (GRAY, 1828) en la relocalización como estrategia de conservación y manejo de la biodiversidad. Unpublished graduate thesis. Universidad Católica de Temuco.
Riley, J. & Edwards, P. (1998). Statistical aspects of aquaculture research: pond variability and pseudoreplication. Aquac. Res., 29(4), 281-288.
Simone, L. R. L. (1994). Anatomical characters and systematics of Anodontites trapesialis (Lamarck, 1819) from South America (Mollusca, Bivalvia, Unionoida, Muteloida). Studies on Neotrop. Fauna Environm., 29(3), 169-185.
Smart, T. S., Riley, J., & Haylor, G. (1997). Eliminating pond differences with cross-over designs. Aquac. Res., 28(8), 621-627.
Smart, T. S., Riley, J., & Edwards, P. (1998). Statistical aspects of aquaculture research: sample sizes for pond experiments. Aquac. Res., 29, 373-379.
Yeager, M. M., Cherry, D. S., & Neves, R. J. (1994). Feeding and burrowing behaviors of juvenile rainbow mussels, Villosa iris (Bivalvia: Unionidae). J. N. Am. Benthol. Soc., 13(2), 217-222.
