Rev. Mar. Cost. ISSN 1659-455X. Vol. 3 111-125, Diciembre 2011.
DOI: https://doi.org/10.15359/revmar.3.9
CHEMICAL AND FUNCTIONAL CHARACTERIZATION OF ANTIMICROBIAL METABOLITES ISOLATED FROM ASCIDIAN RHOPALAEA BIRKELANDI
QUÍMICA Y CARACTERIZACIÓN FUNCIONAL DE LOS METABOLITOS ANTIMICROBIANOS AISLADOS DE ASCIDIA RHOPALAEA BIRKELANDI
Maribel Cordero1, Henry Borbón1*, Félix R. Román2, Luis Morell2, Rigoberto Víquez3,
Luis R. Villegas1, Roy Soto4 and Ilena Vega4
1 Laboratorio de Investigación y Desarrollo en Tecnología Química, Cátedra de Química Orgánica, Universidad Nacional, Heredia, Apdo. 86-3000, Heredia, Costa Rica. *hborbon_2000@hotmail.com
2 Departamento de Química, Universidad de Puerto Rico, PO BOX 9019, 00681-9019, Mayagüez, Puerto Rico.
3 Programa de Maestría en Ciencias Marinas y Costeras, Universidad Nacional, Heredia, Apdo. 36-3000, Heredia, Costa Rica.
4 Laboratorio de Productos Naturales y Ensayos Biológicos, Cátedra de Química Orgánica, Universidad Nacional, Heredia, Apdo. 86-3000, Heredia, Costa Rica
Recibido 30-III-2011
Aceptado 18-VIII-2011
ABSTRACT
Marine ecosystems have a very large diversity of resources, most of them still partially unknown, and a few others exploited for development of new industrial and toxicological products. The objective of this investigation was to determine whether the acetone extract of the ascidia R. birkelandi from the Pacific coast of Costa Rica showed qualitative antimicrobial activity against the S. aureus bacteria and the G. candidum fungus, and to verify their main secondary metabolites in the active extract using chromatographic and spectroscopic techniques. Ascidians were collected at Tambor, Guanacaste, Costa Rica, between December 2007 and March 2008. Activity against the Gram positive bacteria and fungi was evaluated using ethanolic (95%) and acetonic extracts. Both extracts showed activity against G. candidum; however, only the acetonic extract showed activity against S. aureus. A coumarin and a hydroxyanthraquinone were isolated from a crude extract of R. birkelandi as metabolites present in the active fraction. Purification and isolation were performed by chromatographic techniques and solid phase extraction. Structural information was obtained by spectroscopic analyses: Ultraviolet (UV-Visible), Fourier Transform Infrared (FT-IR), Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS), Gas Chromatography Mass Spectrometry (GC/MS), Proton Nuclear Magnetic Resonance (1HNMR), and Carbon-13 Magnetic Resonance (13C-NMR). Further studies are recommended for characterization and quantification of the active components of this extract and the possible elucidation of the mechanisms of action.
Keywords: Antibiotic activity, ascidians, biological activity, bioactive extracts.
RESUMEN
Los ecosistemas marinos poseen una gran cantidad de recursos, muchos de ellos todavía desconocidos y muy pocos de ellos explotados para el desarrollo de nuevos productos industriales y toxicológicos. Los objetivos de esta investigación fueron: determinar si el extracto acetónico de la ascidia R. birkelandi de la costa pacífica de Costa Rica presenta actividad antimicrobial cualitativa contra la bacteria S. aureus y el hongo G. candidum, y determinar por técnicas cromatográficas y espectroscópicas los metabolitos secundarios principales presentes en el extracto activo. Se recolectó la ascidia en la zona de Tambor en la provincia de Guanacaste, Costa Rica, entre los meses de diciembre del 2007 y marzo del 2008. Se realizaron extractos crudos en acetona y etanol al 95% para evaluar la actividad contra una bacteria Gram positiva y un hongo. Ambos extractos presentaron actividad contra G. candidum, pero solo el extracto acetónico mostró actividad contra S. aureus. Se logró aislar y caracterizar una cumarina y una hidroxiantraquinona del extracto crudo de R. birkelandi como metabolitos presentes en la fracción responsable de la actividad biológica presentada. La purificación y el aislamiento fueron llevados a cabo con técnicas cromatográficas y de extracción en fase sólida. La información estructural fue obtenida por análisis espectroscópicos: Ultravioleta (UV-Visible), Infrarrojo con Transformada de Fourier (FT-IR), Cromatografía Líquida acoplada a Espectrometría de Masas (LC/MS/MS), Cromatografía de Gases acoplada a Masas (GC-MS), Resonancia Magnética Nuclear de Protón (1H-NMR) y Resonancia Magnética de Carbono 13 (13C-NMR). Se recomiendan estudios futuros para la caracterización y la cuantificación de los principios activos de este extracto y una posible elucidación de sus mecanismos de acción.
Palabras claves: Actividad antibiótica, ascidias, actividad biológica, extractos bioactivos.
INTRODUCTION
It has been confirmed that secondary metabolites are a source of biologically active natural products with various functions, including antibacterial, antifungal, antiviral, antineoplastic, anticancer, anticoagulant, cytotoxic, hypocholesterolemic, antihelmintic, insecticide, neurotoxic, and molluscicidal activities, acting also as inhibitors and plant growth promoters (AL-Zereini, 2006). This range of properties allows these compounds to have great potential for industrial application. The extract or infusion of the compound, which has been identified to have a specific biological activity, can be used either alone or directly as a foundation in the synthesis of organic compounds. It can also be used within the structure of its active sites, in order to develop new substances with predetermined properties (González & Silva, 2001). In addition, chemical research related to the isolation, biosynthesis and structural elucidation of new natural compounds has contributed to pharmaceutical and agricultural progress, for example, the treatment of diseases, pests and the development of new chemicals (AL-Zereini, 2006).
According to AL-Zereini (2006), in 2003 approximately 6% of marine natural products were obtained from tunicates. The major problem has been their identification due to their complex taxonomy. As reported by Davidson (1993), ascidians, for instance, showed a knack of biosynthesized metabolites derived from amino acids, with over 80% of identified nitrogenous compounds, which included peptides (linear and cyclic), several alkaloids and other significant substances, such as terpenoids, isoprenoids, hydroquinones, fatty acid derivatives, and polyethers (McClintock & Baker, 2001).
However, few studies have been done on urochordates (tunicates) and sea squirts. In countries like Cuba, Puerto Rico, Brazil, the United States, and Spain, research has been conducted showing the biological and chemical taxonomy of these organisms (Proksch et al. 2003). Nevertheless, studies related to the activity and isolation of compounds from sea squirts in Costa Rica have been limited due to the difficulty of classification of these types of marine organisms (Davies-Coleman & Beukes, 2004).
In this context, considering international contributions with respect to work performed on the Ascidiacea biological class as a source of bioactive chemicals, the objective of this project was to evaluate its potential antibiotic activity to make a diagnosis of the chemistry related to the ascidian extract of Rhopalaea birkelandi. In addition, the study is aimed at qualitatively characterizing the chemical nature of the compounds isolated from the extract or the fraction with the highest antimicrobial activity against pathogens Staphylococcus aureus and Geotrichum candidum.
MATERIALS AND METHODS
Material collection
Ascidians of the genus Rhopalaea were collected manually by scuba divers at depths between 2 and 15 m in the Northern Pacific area of Costa Rica, specifically in the Tambor area, province of Guanacaste, Latitude 9° 43’ 0.663’’ (N), Longitude 85° 0’ 58.3524’’ (W), during the dry season between December 2007 and March 2008, using a simple random sampling. They were recognized based on physical characteristics of the genus described by Carballo (2006).
Material was cleaned with seawater to remove sand and epiphytic organisms, separated by species and placed in transparent polypropylene bags, which were drained of excess water by gravity. Samples were frozen for transport to prevent decomposition, loss of concentration and their normal activity, as recommended by Caccamese et al. (1980) and Rao and Parekh (1981).
Compound extraction
A portion of each sample was fixed in formalin: glycerin (9:1) for subsequent taxonomic confirmation following what was mentioned by Van Name (1945). The sample was washed with distilled water and cleaned of epiphytes under a dissecting microscope. Excessive water was removed by placing the samples on absorbent paper for a day (Pesando & Caram, 1984) and by freeze drying them.
Approximately 10 g of lyophilized material was crushed with a mortar and pestle and homogenized for 72 hours at room temperature with 30 mL of solvent for extraction (acetone and ethanol) with occasional agitation. The material was filtered using a Büchner funnel and a Whatman filter paper # 1. The remaining extract was centrifuged at 3400 rpm (1000xg) for 15 minutes to remove any solid residue not retained on the filter (Rao & Parekh, 1981). The supernatant was concentrated under vacuum in a Büchi R-110 model rotary evaporator, with a maximum temperature of 95°F (35°C) to obtain an extract with viscous consistency. The crystals obtained were suspended in 10 mL of extraction solvent (Wahl et al. 1994).
Antibacterial and antifungal bioassays
Bioassay tests were conducted in sterile disposable Petri dishes. A total of 15 mL of Staphylococcus #110 agar was added and inoculated with 0.1 mL of S. aureus strain (Gram positive). In addition, 15 mL of Potato Dextrose Agar were used to inoculate the G. candidum fungus (Fungi), and the medium was left to solidify at room temperature. The disks used to inoculate the extract were 6 mm Whatman filter paper No. 42, which were loaded aseptically with 0.1 mL of the extracts. The solvent was evaporated. The disks were immediately placed in culture media over a target with the extraction solvent as negative control. The dishes were left incubating at 98.6°F (37°C) for 48 hr in a sterile room. Inhibition halos were measured with a vernier caliper (De Lara-Isassi et al. 1996; Jorgensen et al. 1999). The drugs used as reference standards for these assays were Keflex® cephalosporin antibiotic, which is recommended by the Costa Rican Social Healthcare to treat infectious diseases caused by the S. aureus pathogen, and Miconazole Nitrate® 2%, which is a commercial antifungal with high spectrum against the G. candidum fungus. They represent the positive control bioassays in the methodology described above. Based on the results obtained in the bioassay, the extract selected was the one showing the highest mean inhibition zone.
Purification of the metabolite
The crude extract was analyzed by thin layer chromatography (TLC), using silica gel as the stationary phase and solvent mixtures of hexane/ethyl acetate and hexane/THF as the mobile phase. In all cases, TLC method development such as vanillin in H2SO4, FeCl3 1% and ammonia were used. The sample from the residual acetone was placed on top of the column and the separation began with a mobile phase of hexane. Polarity was gradually increased using hexane/ethyl acetate mixtures. Similarities in the retention factor (Rf) resulted in 14 fractions, which were analyzed comparatively with thin layer chromatography on silica gel using ultraviolet light, and then revealed with vanillin.
Every fraction was subjected to the bioassay described above to determine the inhibitory activity on the growth of bacteria. The active fraction was fractionated again with a chromatography column with smaller diameter and greater length. Silica gel was the stationary phase and hexane and THF the mobile phase. The fractions of the active fraction were first treated by solid phase extraction (SPE) with a normal phase (silica) using hexane to wash impurities, and then separated with chloroform, acetone, and ethanol. Five fractions were obtained with this method.
Identification of the metabolites
The fractions were analyzed with high performance liquid chromatography (HPLC) using an Agilent 1100 Series Diode-array detector (DAD) equipped with an MS/MS Bruker Esquire 6000. Metabolites in the purified fractions were identified in an analytical column, Supelcosil LC-NH2, 25 cm x 4.6 mm, 5 μm, using an isocratic mobile phase consisting of methanol/H2O 50:50. The analysis was carried out at a wavelength of 254 nm and 280 nm, with an operating time of 10 minutes at a flow rate of 1.0 mL/min. Solvents used in the mobile phase were filtered through membrane filters of 0.45 μm in each analysis. The UV-Visible spectrum was obtained directly in the analysis at a wavelength of 200 to 800 nm by high performance liquid chromatography (HPLC) using the Agilent 1100 Series Diode-array detector (DAD). Compounds were dissolved in methanol before the analysis. The FT-IR spectrum of the isolated compounds was obtained using the Bruker IFS 66 V/S computer model. The percentage transmittance was measured against the wave number from 3600 to 600 cm-1 in a KBr pellet. The mass spectrum was obtained with an MS/MS Bruker Esquire 6000 Electrospray API-ES through direct injection in positive mode. The scan was performed from 50 m/z to 200 m/z (sample A), and to 300 m/z (sample B). Samples were dissolved in acetone. NMR spectra were obtained with a Bruker Avance 500 MHz NMR. In this case, sample A was dissolved in deuterochloroform and sample B in deuterated dimethyl sulfoxide.
RESULTS AND DISCUSSION
Antibacterial activity
The crude acetone extract of the ascidian R. birkelandi showed the highest positive activity against the G. candidum fungus. The average radius of inhibition against the G. candidum fungus was 14.5 ± 0.5 mm, while the average radius of inhibition yielded against the S. aureus bacterium was 22.0 ± 0.5 mm. According to Rojas et al. (2006), inhibition against G. candidum is considered intermediate because it ranges between 13 and 17 mm, while inhibition against S. aureus is significant because it is greater than 17 mm. The commercial Keflex® inhibition was 25.0 ± 0.5 mm, which agrees with the data reported by Rojas et al. (2006).
The crude ethanol extract of the ascidian R. birkelandi only showed activity against the G. candidum fungus. Its average radius of inhibition was 2.5 ± 0.5 mm, which is considered moderate as it ranges between 2-13 mm. On the contrary, the effect against S. aureus was practically zero, since the ethanol extract showed no activity. Commercial Miconazole Nitrate 2% showed an average radius of inhibition of 15.0 ± 0.5 mm against the G. candidum fungus (Table 1).
A total of 14 fractions were collected from the acetone extract of the chromatography column. Numbers 1, 2, and 3 correspond to inhibition against S. aureus. Fractions 2 and 3 showed inhibition areas of 10.0 ± 0.5 mm and 8.0 ± 0.5 mm, respectively. Both showed a strong inhibition similar to Keflex® that presented an inhibition of 25.0 ± 0.5 mm. A negative control of acetone was performed in the experiment.
The inhibition results obtained are consistent with the literature, as stated by Hooper et al. (1995), who reported the antibacterial activity of the ascidian Pseudodistoma sp against S. aureus. Liu et al. (2005) reported the compound lissoclibadin 1 isolation of the Lissoclinum cf. badium species with antibacterial activity. The metabolite of styelins A-E isolated from the solitary ascidian Styela clava showed inhibitory activity against bacteria, which are human pathogens such as S. aureus and Escherichia coli, according to Lehrer et al. (2003). Furthermore, Makarieva et al. (1995) reported the ascidian Polycitor sp. with potent inhibitory activities against human pathogens such as S. aureus and Bacillus subtilus.
The results of the inhibitory effect were greater in the acetone extract than the ethanol extract for both organisms. This indicates that the metabolites responsible for such effect are soluble in this solvent, which agrees with the results from Rao & Parekh (1981); Vidyavathi & Sridhar (1991), and De Lara-Issasi & Ponce-Márquez (1991), who indicate that bioassay conducted with marine organisms using acetone as solvent extraction showed a better response as antibiotics in general than those performed with ethanol or any other solvent with higher polarity.
The fractions obtained by column chromatography from the crude acetone extract of the ascidian R. birkelandi were tested against S. aureus, obtaining 3 positive fractions out of 14. The results showed slightly less antibacterial activity in these individual fractions than in the crude extract. This observation is possibly due to the synergic action of secondary metabolites in the crude extract. Future studies should calculate the lowest concentration of each compound (quantitative analysis) that would inhibit the visible growth of the microorganism. This aspect is very important to confirm resistance of microorganisms to an antimicrobial agent and also to monitor the activity of new antimicrobial agents.
Purification and isolation of metabolites
Based on the results of the antibacterial activity, the purification process of fraction 2 was achieved using column chromatography and solid phase extraction, and isolation and purification was conducted using HPLC. Identification of purity was carried out at wavelengths of 258 and 280 nm using a diode array detector, which is able to detect two metabolites in pure form. HPLC results showed a retention time of 5.640 min in the chromatogram of compound A, fraction 2, and a retention time of 6.769 min in the chromatogram of compound B of the same fraction.
The UV-Visible spectrum of isolated purified compound A presented two maximum absorption bands, approximately 220 nm and 290 nm. These are characteristic wavelengths of heterocyclic compounds with aromatic rings attached to oxygen because the system conjugation allows carrying out transitions such as π → π * and n → π * and a bathochromic shift of the bands occurs (Silverstein et al. 2004).
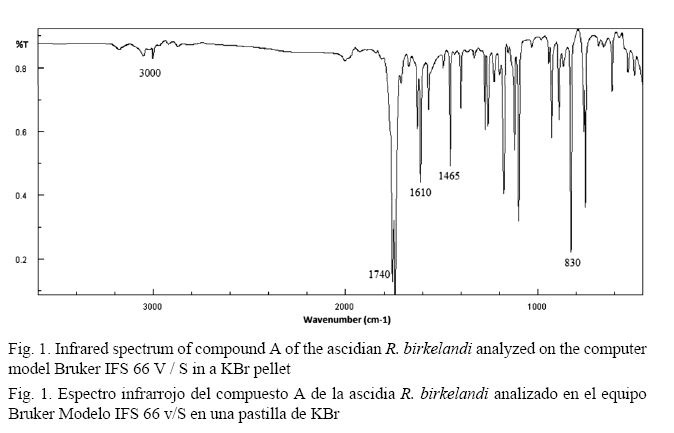
The Fourier Transform Infrared Spectrum (FT-IR) of compound A (Fig. 1) does not show significant bands in the 3500 - 2000 cm-1 region, ruling out the presence of OH groups (Pavia et al. 2009). The lengthening of the double bonds of the aromatic ring is represented by signals in the 1610 cm-1 and 1465 cm-1 region. The small band of approximately 3000 cm-1 occurs due to the stretching of the CH bond of the sp2 hybridized carbons belonging to the aromatic ring and forming an alkene outside this ring. The band near 1740 cm-1 represents a characteristic carbonyl band due to the C=O stretching. The typical long and intense band in 830 cm-1 is the cis configuration of an alkene (Pavia et al. 2009).
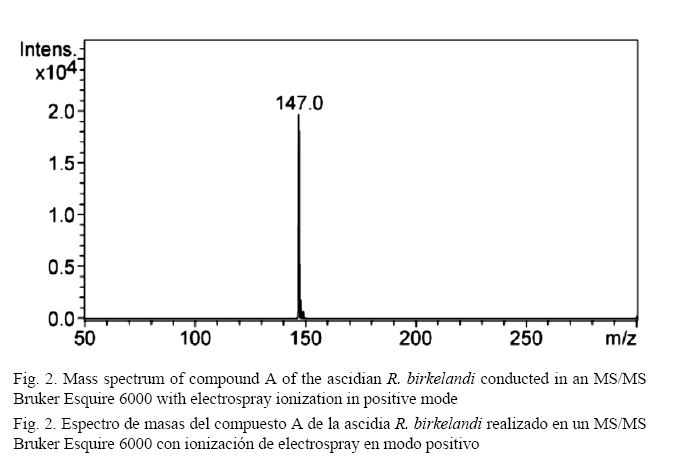
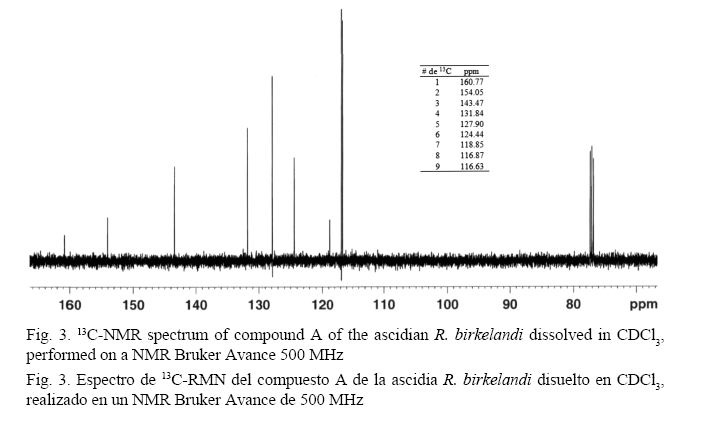
As shown in Fig. 2, the mass spectrum shows a base peak at 147 m/z, and the isotopic ratios of x+1 and x+2 provide information to obtain the experimental molecular formula C9H6O2. The analysis of the 13C spectrum (500 MHz) (Fig. 3) confirms the molecular formula suggested in the mass analysis by presenting 9 carbon signals and several of them downfield: the first of them was 160.77 ppm, which is a characteristic value for carbons in an ester carbonyl group. Signals at 154.05 ppm, 143.47 ppm, 131.84 ppm, 127.90 ppm, 124.44 ppm, and 118.85 ppm correspond to the usual values for carbons in an aromatic ring. Signals at 116.87 ppm and 116.68 ppm are also within the range for carbon aromaticity and could correspond to the values of carbons belonging to a double bond in an alkene. The presence of oxygen and a carbonyl group on the structure suggests a shift toward lower field in those carbons that are directly linked to this group and this atom (Pavia et al. 2009).
The signals displayed by the 13C-NMR spectrum are consistent with the 1H-NMR spectrum. The analysis of the 1H-NMR spectrum (500 MHz) (Fig. 4) shows several signals at low field: between δ 7.49 - 7.55 (2H) and δ 7.27 - 7.34 (2H). There is evidence of characteristic hydrogen signals attached to an aromatic ring, which are deshielded by the anisotropic field generated by π electrons in the ring system (Pavia et al. 2009). The main splittings show a triplet and a doublet for each of the groups of signals, indicating an ortho substitution on the ring. The coupling constant (3J = 8.51 Hz) confirms the ortho positions between hydrogens. Enlargement of the signal shows a more complex splitting of the second order, usually in substituted aromatic rings (Pavia et al. 2009). A signal at δ 7.73 (d, 1H) is due to a hydrogen coupling with another hydrogen neighbor (signal at δ 6.43 (d, 1H)), both corresponding consequently to an alkene with hydrogen atoms in cis position (3J = 9.58 Hz). The cis alkene belongs to a ring attached to the aromatic ring of the molecule. The downfield shift of hydrogen is due to deshielding by the anisotropic field of the aromatic ring and the delocalized electron density resonance with the carbonyl group attached to the alkene and changes in its hybridization (Pavia et al. 2009).

In the case of the isolated and purified compound B, the UV-Visible spectrum has two maximum absorption bands around 250 nm and 295 nm, which are characteristic wavelengths of heterocyclic compounds of aromatic rings attached to oxygen. This conjugation system allows for transitions π → π * and n-type → π * (Silverstein et al. 2004).
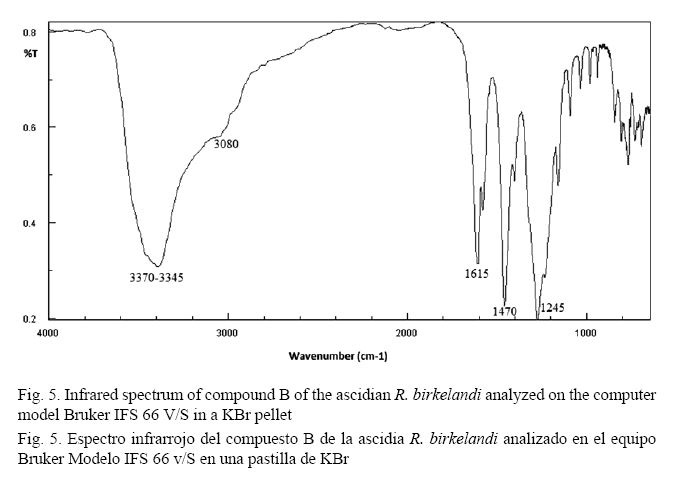
The Fourier Transform Infrared Spectrum (FT-IR) of compound B (Fig. 5) presents a band at 3080 cm-1. This band is the CH stretching of sp2 hybridized carbon atoms belonging to aromatic rings. The lengthening of the double bonds of the aromatic ring is represented by signals in the region at 1580 cm-1 and 1470 cm-1. The band close to 1615 cm-1 represents a characteristic band due to conjugated carbonyl C=O stretching. The middle band around 3370 cm-1 - 3345 cm-1 is characteristic of phenolic OH, which is confirmed by the band at 1245 cm-1 (Pavia et al. 2009).
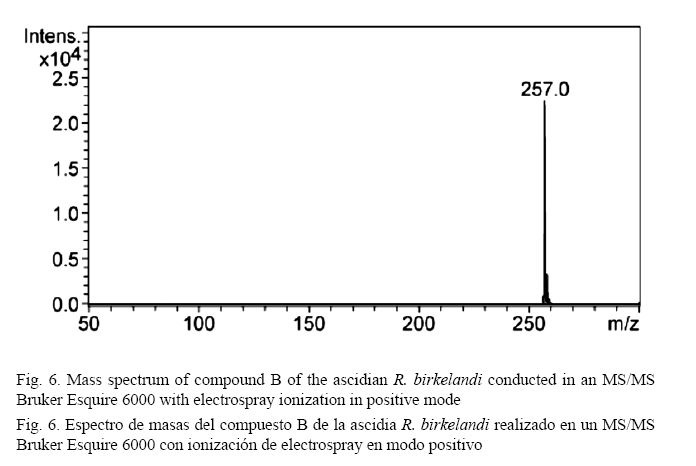
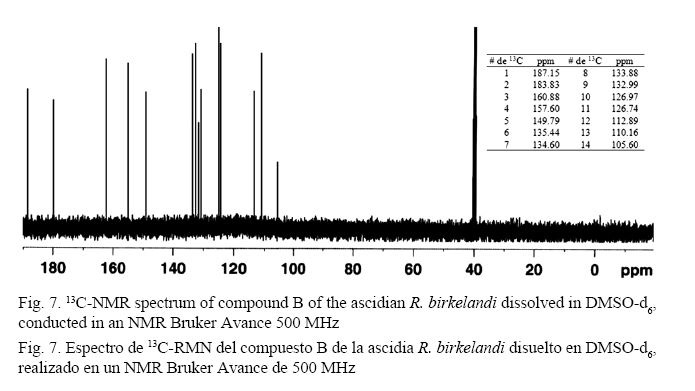
As shown in Fig. 6, the mass spectrum shows a base peak at 257 m/z and isotopic ratios of x+1 and x+2 to estimate the molecular formula C14H8O5. The analysis of the 13C-NMR spectrum (500 MHz) (Fig. 7) confirms the molecular formula suggested by mass spectrometric analysis to provide signals for 14 carbons. There are two low field signals at 187.15 ppm and 183.83 ppm. These values are common for carbons belonging to a carbonyl group of ketones or aldehydes and are close to the values for acids and esters (Pavia et al. 2009). The signals between 160.88 ppm and 105.60 ppm, which are common for aromatic ring carbons, suggest the presence of two rings in the structure and 12 signals indicating no plane of symmetry for these rings. The base value for these carbons is 128.5 ppm (Pavia et al. 2009); however, the downfield shift of carbons 160.88 ppm, 157.60 ppm, and 149.79 ppm of the ring suggests the possible presence of the hydroxyl group attached to these carbons, whose displacement is comparable to those reported in similar phenolic models. The positions of the substituents in the aromatic ring are related to the chemical shifts of carbon 13 (ipso, ortho, meta and para) observed in the spectrum (Pavia et al. 2009).
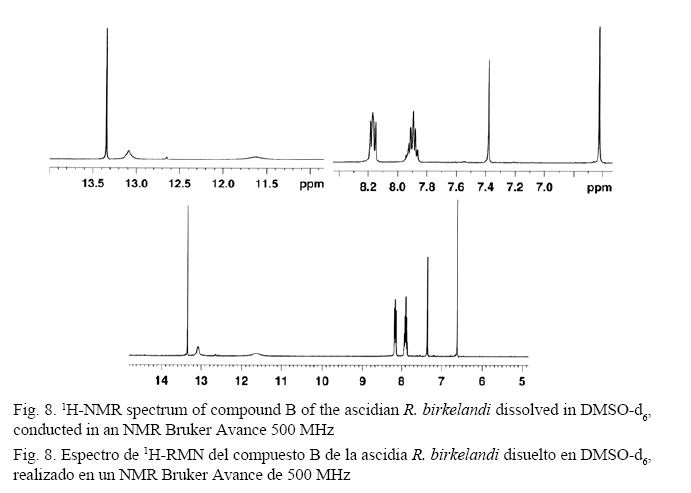
The signals displayed by the 13C-NMR spectrum are consistent with the 1H-NMR spectrum. The analysis of the 1H-NMR spectrum (500 MHz) (Fig. 8) shows a downfield signal at δ = 13.35 ppm (s), which is attributed to a hydrogen attached to the oxygen in phenol in the β position with respect to the carbonyl group of the molecule. The proton interacts by hydrogen bond with the oxygen in the carbonyl group. This phenomenon shifts the proton of phenol far downfield (Pavia et al. 2009). Other low-field signals at δ = 8.15 - 8.19 ppm (t, 2H) and δ = 7.87 - 7.94 ppm (m, 3H) are distinctive of the hydrogens attached to an aromatic ring, which are deshielded by the anisotropic field generated by π electrons in the ring system (Pavia et al. 2009). The main splittings show a triplet and a multiplet for each of the groups of the signals, indicating a complex second order substitution in the rings. The coupling constant (3J = 7.65 Hz) between hydrogen ortho positions confirmed it. Enlargement of the signals shows the second order complex cleavage, usually common for these ring system substitutions (Pavia et al. 2009).
A signal at δ = 7.37 ppm (s, 1H) and another at δ = 6.63 ppm (s, 1H) can be attributed to the proton of a hydroxyl group attached to the aromatic ring of the molecule, which does not interact with any other functional group of the compound. The downfield shift of hydrogen is due to deshielding by the anisotropic field of the ring and to resonance that delocalizes density of oxygen electrons and changes its hybridization (Pavia et al. 2009).
Evidence suggests that compound A isolated from the ascidian R. birkelandi has the chemical basis of coumarins or derivatives, which have been reported in literature as compounds based on structures with cytotoxic activity (Joullié et al. 2003; Reddy et al. 1999). Joullié et al. (2003) reported similar didemnin B fluorescent metabolites, tamandarin and its potential chemical defense mechanisms in Didemnidae tunicates. These compounds showed a strong antipredatory relationship, inhibiting protein synthesis, initiating apoptosis and reducing cell proliferation. Reddy et al. (1999) found a new inhibitor of HIV-1 integrase named Lamellarin α 20-sulfate. The coumarin basis structure, which has been found in previous studies, is an active integrase inhibitor. Coumarins have been associated with some properties in organisms, playing a defensive role. Some of them have food rejection (antifeedant), antimicrobial, UV-grabbing and inhibitory germination properties (O’Neil, 2006). This suggests a possible role of the compound found with the reported cytotoxic activity against S. aureus and G. candidum.
In the case of compound B isolated from the ascidian R. birkelandi in acetone extract, spectroscopic evidence suggests a chemical basis of a hydroxyanthraquinone, which has been reported in literature as compounds present in some ascidians (McClintock & Baker, 2001). Poumale et al. (2006) discovered two new anthraquinones isolated from Streptomyces body of the ascidian Ecteinascidia sp. being identified as 8-hydroxy-3-methoxy-1-propilanthraquinone and 3,8-dihydroxy-1-propylanthraquinone, after intense spectroscopic analysis. Some of the derivatives of the compounds were tested for biological activity yielding positive results.
Another Streptomycete chemical study of a bacterium isolated from a tunicate associated with tropical Ecteinascidia turbinate showed two metabolites with antibacterial bisantraquinona base (Socha et al. 2006). Powerful antibacterial properties against S. aureus and Enterococcus faecalis and activity against HCT-116 cells were detected. Issa et al. (2003) found three Floresolides: A 627, B 628 and C 629, which have a chemical basis of hydroquinones with cytotoxic properties. These floresolides were isolated from the ascidian Aplidium sp. from Flores Island, Portugal. These results may also suggest a possible role of the compound found with the reported cytotoxic activity against S. aureus and G. candidum.
Several marine-derived compounds are currently in clinical trials and it is likely that many more will be included in the trails as more scientists turn to the sea for these biotechnological uses. Cephalosporins are a good example of antimicrobial agents since they owe their origin to a marine source. Cephalosporin C was isolated from the marine fungus Cephalosporium acremonium. A semisynthetic derivate of this, cephalothin sodium, has been widely used as an antibiotic (Murti & Agrawal, 2010). Recent research demonstrates that the marine ecosystem is a tool to identify new cellular targets for therapeutic intervention. Marine-derived pharmaceuticals require more basic and applied research in order to bring this resource to the same level as patented pharmaceutical products and gain acceptance from the medical community. An interaction between researchers, the pharmaceutical sector and government regulating agencies is important to incorporate this challenging new tool into the clinical medicine.
ACKNOWLEDGEMENTS
We would like to thank the Coordinator of Environmental Chemistry, Félix R. Román, from the University of Puerto Rico (Mayaguez Campus) for his assistance and support in the implementation of this project.
REFERENCES
AL-Zereini, W. (2006). Natural products from marine bacteria. (Unpublished doctoral dissertation). University of Kaiserslautern, Germany.
Caccamese, S. R., Azzolina, G., Furnari, M., Cormaci, M., & Grasso S. (1980). Antimicrobial and antiviral activities of extracts from Mediterranean algae. Bot. Mar., 23, 285-288.
Carballo, J. L. (2006). Aportación al conocimiento de la fauna de Ascidias del litoral Pacífico de México. (Informe final SNIB-CONABIO proyecto No. BC005). Universidad Nacional Autónoma de México, Mexico D.F.
Davidson, B. S. (1993). Ascidians: producers of amino acid-derived metabolites. Chem. Rev., 93(5), 1771-1791.
Davies-Coleman, M. T. & Beukes, D. R. (2004). Ten years of marine natural products research at Rhodes University. S. Afr. J. Sci., 100(11, 12), 539-544.
De Lara-Isassi, G. & Ponce-Márquez, M. E. (1991). Detección de la actividad antibacteriana de algunas algas de Playa Paraíso, Veracruz, México. BIOTAM, 3(1), 20-26.
De Lara-Isassi, G., Álvarez-Hernández, S., & Lozano-Ramírez, C. (1996). Actividad antibacteriana de algas marinas de Oaxaca, Pacífico Tropical Mexicano. Rev. Biol. Trop., 44(2), 895-898.
González, F. & Silva, M. (2001). Biodiversidad Química de Macroalgas Marinas. In K. Alveal & T. Antezana (Eds.), Sustentabilidad de la biodiversidad un problema actual bases científico-técnicas, teorizaciones y proyecciones (pp.415-496). Concepción, Chile: Universidad de Concepción.
Hooper, G. J., Davies-Coleman, M. T., & Coetzee, P. S. (1995). New antimicrobial C14 and C13 amines from a South African marine ascidian. Nat. Prod. Lett., 6(1), 31-35.
Issa, H. H., Tanaka, J., Rachmat, R., & Higa, T. (2003). Floresolides, new metacyclophane hydroquinone lactones from an ascidian, Aplidium sp. Tetrahedron Lett., 44(6), 1243-1245.
Jorgensen, J. H., Turnidge, J. D., & Washington, J. A. (1999). Antibacterial susceptibility tests: dilution and disk diffusion methods. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, & R. H. Yolken (Eds.), Manual of Clinical Microbiology (pp.1526-1543). Washington, DC: American Society for Microbiology.
Joullié, M. M., Leonard, M. S., Portonovo, P., Liang, B., Ding, X., & La Clair, J. J. (2003). Chemical defense in ascidians of the didemnidae family. Bioconjug. Chem., 14(1), 30-37.
Lehrer, R. I., Tincu, J. A., Taylor, S. W., Menzel, L. P., & Waring, A. L. (2003). Natural peptide antibiotics from tunicates: structures, functions and potential uses. Integr. Comp. Biol., 43, 313-322.
Liu, H., Fujiwara, T., Nishikawa, T., Mishima, Y., Nagai, H., Shida, T., Tachibana, K., Kobayashi, H., Mangindaan, R. E. P., & Namikoshi, M. (2005). Lissoclibadins 1-3, three new polysulfur alkaloids, from the ascidian Lissoclinum cf. badium. Tetrahedron Lett., 61(36), 8611-8615.
Makarieva, T. N., Stonik, V. A., Dmitrenok, A. S., Grebnev, B. B., Isakov, V. V., Rebachyk, N M., &. Rashkes, Y. W. (1995). Varacin and three new marine antimicrobial polysulfides from the far-eastern ascidian Polycztor sp. J. Nat. Prod., 58(2), 254-258.
McClintock, J. B. & Baker, B. J. (2001). Secondary metabolites from Antarctic marine organisms and their ecological implications. In J. B. McClintock & B. J. Baker (Eds.), Marine Chemical Ecology (pp. 23-25). Florida, USA: CRC Press.
Murti, Y. & Agrawal, T. (2010). Marine derived pharmaceuticals-development of natural health products from marine biodiversity. Int. J. ChemTech Res., 2(4), 2198-2217.
O’Neil, M. J. (2006). The Merck index- an encyclopedia of chemicals, drugs, and biologicals. (14th. ed.) New Jersey, USA: Merck.
Pavia, D. L., Lampman, G. M., Kriz, G. S., & Vyvyan, J. R. (2009). Introduction to spectroscopy. (4rd. ed.). Belmont, CA, USA: Brooks Cole, Cengage, Learning.
Pesando, D. & Caram, B. (1984). Screening of marine algae from the French Mediterranean coast for antibacterial and antifungal activity. Bot. Mar., 27(8), 381-386.
Poumale, H. M. P., Ngadjui, B. T., Helmke, E., & Laatsch, H. (2006). New anthraquinones from a marine Streptomycetes sp. -Isolation, structure determination and biological activities. Z. Naturforsch., 61b(11), 1450-1454.
Proksch, P., Ebel, R., Edrada, R. A., Schupp, P., Lin, W. H., Wray, V., & Steube, K. (2003). Detection of pharmacologically active natural products using ecology. Pure Appl. Chem., 75(2, 3), 343-352.
Rao, P. S. & Parekh, K. S. (1981). Antibacterial activity of Indian seaweed extracts. Bot. Mar., 24(11), 577-582.
Reddy, M. V., Rao, M. R., Rhodes, D., Hansen, M. S., Rubins, K., Bushman, F. D., Venkateswarlu, Y., & Faulkner, D. J. (1999). Lamellarin α 20-sulfate, an inhibitor of HIV-1 integrase active against HIV-1 virus in cell culture. J. Med. Chem., 42(11), 1901-1907.
Rojas, N., Chaves, N., & García, F. (2006). Bacteriología diagnóstica. San José, Costa Rica: Editorial UCR.
Silverstein, R. M., Webster, F. X., & Kiemle, D. J. (2004). Spectrometric identification of organic compounds. (7th ed.). New York, USA: Wiley.
Socha, A. M., García, D., Scheffer, R., & Rowley, D. C. (2006). Antibiotic bisanthraquinone produced by a Streptomycetes isolated from a cyanobacterium associated with Ecteinascidia turbinata. J. Nat. Prod., 69(7), 1070-1073.
Van Name, W. G. (1945). The North and South American ascidians. Bull. Amer. Mus. Nat. Hist., 84, 1-476.
Vidyavathi, N. & Sridhar, K. R. (1991). Seasonal and geographical variation in the antimicrobial activity of seaweeds from the Mangalore coast of India. Bot. Mar., 34(4), 279-284.
Wahl, M., Jensen, P. R., & Fenical, W. (1994). Chemical control of bacterial epibiosis on ascidians. Mar. Ecol. Prog. Ser., 110, 45- 57.

Revista Ciencias Marinas y Costeras está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.