Rev. Mar. Cost. ISSN 1659-455X. Vol. 5: 107-117, Diciembre 2013.
Preliminary assessment of small scale bacterial depuration of Crassostrea gigas and Anadara spp., Gulf of Nicoya, Costa Rica
Evaluación preliminar de la depuración bacteriana a pequeña escala de Crassostrea gigas y Anadara spp., Golfo de Nicoya, Costa Rica
Luis A. Vega Corrales1*, Carolina Marín Vindas1, Oscar Pacheco Prieto1 & Gerardo Zúñiga Calero1
1 Estación de Biología Marina Juan Bertoglia Richards, Universidad Nacional, Costa Rica. luis.vega.corrales@una.cr*
Recibido 30 IV 2013
Aceptado 05 IX 2013
ABSTRACT
Bivalve molluscs are sold without any sanitary control in Costa Rica, which represents a public health risk due to the possible accumulation of pathogenic bacteria. Small scale bacterial depuration treatments were preliminarily evaluated on Crassostrea gigas and Anadara spp. from the Gulf of Nicoya, Costa Rica, through the use of a recirculating system with UV irradiation. The levels of Escherichia coli in the water were determined using the MPN method. The MPN of E. coli and the presence of Salmonella spp., Vibrio parahaemolyticus and V. cholerae were determined in molluscs. Results confirm the effectiveness of the E. coli depuration system design in C. gigas and Anadara spp. The MPN of E. coli in the molluscs was reduced between 79% and 100% after 24 h. None of the other types of bacteria were found in the samples analyzed. This is the first C. gigas depuration study in Costa Rica and the first report concerning UV depuration for bacterial indicators of fecal contamination in Anadara spp. This research serves as a basis for the implementation and improvement of mollusc purification conditions in the country. It is recommended to monitor extraction and harvesting areas, as well as to implement the depuration of bivalve molluscs so that traditional producers can offer an innocuous product with added value.
Keywords: Gulf of Nicoya, bacterial depuration, Crassostrea gigas, Anadara spp., E. coli.
RESUMEN
Los moluscos bivalvos son comercializados sin ningún control sanitario en Costa Rica; por tanto, representan un riesgo para la salud pública, debido a que pueden acumular bacterias patógenas. Se evaluaron preliminarmente tratamientos para la depuración bacteriana a pequeña escala de Crassostrea gigas y Anadara spp. en el Golfo de Nicoya, Costa Rica, utilizando un sistema recirculado e irradiado con luz UV. Los niveles de Escherichia coli en el agua se determinaron mediante el método del NMP. A los moluscos se les determinó el NMP de E. coli y la presencia de Salmonella spp., Vibrio parahaemolyticus y V. cholerae. Los resultados confirman la efectividad del diseño del sistema para la depuración de E. coli en C. gigas y Anadara spp. A las 24 h, el NMP de E. coli en los moluscos se redujo entre el 79% y el 100%. No se comprobó la presencia de las demás bacterias en las muestras analizadas. Este es el primer estudio sobre depuración de C. gigas en Costa Rica y el primer reporte de depuración con UV de indicadores bacterianos de contaminación fecal en Anadara spp. Este trabajo sirve de base para implementar y mejorar las condiciones de la depuración de moluscos en el país. Se recomienda monitorear las áreas de extracción y cultivo e implementar la depuración de los moluscos bivalvos para que los productores artesanales puedan ofrecer un producto inocuo y con valor agregado.
Palabras claves: Golfo de Nicoya, depuración bacteriana, Crassostrea gigas, Anadara spp., E. coli.
INTRODUCTION
The harvesting of oysters (Crassostrea gigas) and extraction of mangrove cockles (Anadara spp.), comprised of the species A. tuberculosa and A. similis (MacKenzie, 2001; Silva & Bonilla, 2001) in the Gulf of Nicoya, Costa Rica, generates a considerable source of revenue for small organized groups. However, these organisms are being sold without any sanitary controls, creating a public health risk (Oliveira et al. 2011) due to the possible accumulation of pathogenic bacteria if the extraction and harvesting areas are contaminated by residual waters (Clark, 2001) or if they are handled without proper hygiene regulations (Pouch & Ito, 2001).
Some of the most hazardous bacteria associated to the consumption of bivalve molluscs include Salmonella spp., Escherichia coli, Vibrio parahaemolyticus and V. cholerae, all of which are used as indicators to evaluate the innocuousness of these organisms.
Some species of the Salmonella genus can cause typhoid and paratyphoid fever, as well as gastroenteritis, also caused by E. coli. Both of these bacteria are related to fecal contamination in the extraction and harvesting areas of bivalve molluscs, or the lack of appropriate handling practices (Pouch & Ito, 2001; Lee et al. 2010). Furthermore, although microorganisms of the Vibrio genus are usually found in marine environments, some species are potentially pathogenic for humans, such as V. parahaemolyticus and V. cholerae, which can be transmitted though the ingestion of contaminated water or food (Oberbeckmann et al. 2012).
However, the concentration of these bacteria in bivalve molluscs can be reduced through bacterial depuration (Lee et al. 2010). Controlled purification reduces the number of pathogenic microorganisms present in bivalve molluscs until they can reach an acceptable level for human consumption (Oliveira et al. 2011).
The objective of this study was to evaluate preliminarily small scale bacterial depuration of C. gigas and Anadara spp. in the Gulf of Nicoya, Costa Rica, in order to generate basic information to implement and improve purification conditions of molluscs in the country and provide traditional producers with an alternative to offer a safe product with an added value and no risks to public health.
MATERIALS AND METHODS
Sampling Areas and Treatments
Samples of C. gigas were collected in the harvesting areas of Costa Pájaros (10°5’42’’N and 84°59’4’’W) and Punta Morales (10°4’84’’N and 84°58’37’’W), while samples of Anadara spp. were collected in the mangroves of Chomes (10°2’38’’N and 84°54’47’’ W). Organisms were transported without ice to ensure their survival.
Depuration treatments were performed at the Estación Nacional de Ciencias Marino Costeras (National Coastal Marine Science Station) of Universidad Nacional (ECMAR-UNA), located near the extraction and harvesting areas of the analyzed molluscs. Figure 1 shows the design of the depuration system used. The depuration process was completed for all treatments using a recirculating system in a concrete pond with a capacity of 3 m3, an exchange rate of 5.5 L/s and ultraviolet light irradiation (254 nm) (ULTRADYNAMICS® 8102-LI 30). Organisms were placed in the system inside baskets for 40 h and were not fed during the entire process. Water used was collected from the natural environment.
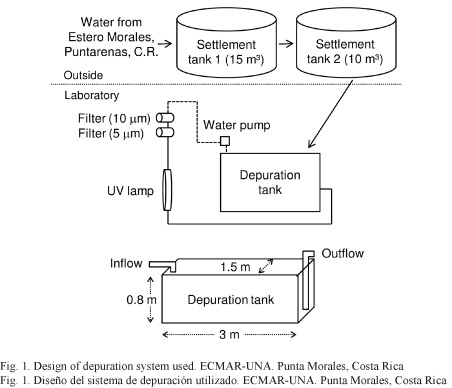
Treatments performed:
Treatment A
Performed in April 2010 with 3 500 oysters, which did not receive any processing before depuration. The water used in the system was irradiated for 36 h before placing the organisms.
The system’s temperature, salinity, and oxygen level were not recorded.
Treatment B
Performed in September 2010 with 3 000 oysters, which did not receive any processing before depuration. The water used in the system was not previously irradiated. Water physicochemical parameters were as follows: 24.9 - 26.4 °C temperature, 22 - 23 PSU salinity and 5.1 - 8.1 mg/L oxygen level.
Treatment C
Performed in August 2010 with 900 cockles washed with potable water before being placed in the system. The water used in the system was irradiated for 39 h before depuration of the organisms. Water physicochemical parameters were as follows: 27.1 - 28.4 °C temperature, 31 PSU salinity and 6.2 - 7.6 mg/L oxygen level.
Treatment D
Performed in November 2011 with 1 170 cockles washed with potable water before being placed in the system. The water used in the system was not previously irradiated. Water physicochemical parameters were as follows: 26.5 - 27.5 °C temperature, 32 PSU salinity and 5.1 - 6.47 mg/L oxygen level.
No replicates of the treatments were performed since oyster and mangrove cockle production nationwide is a artisanal activity and the volume of production varies according to the effort.
Sample Taking
Water samples were collected superficially from the depuration tank before placing the organisms in the system and after treatment. These samples were transported to the lab on ice (approx. 4 °C) and analyzed within a time lapse of 6 h.
Because initial water samples of treatments B and D had not been previously disinfected by the system, they were used to assess the microbiological quality of the water used.
Between 15 and 18 molluscs were sampled aseptically after 0, 8, 16, 24, 32 and 40 h of starting the depuration process. Samples were transported to the lab on ice (approx. 4 °C) and analyzed within a time lapse of 12 h.
Microbiological Analysis
The levels of E. coli in the water were determined through the most probable number (MPN) method with five tubes and three dilutions, as described by Clesceri et al. (1989). During the presumptive phase, each series of five tubes with Lauryl Tryptose Broth (BD®) was inoculated with 10, 1 and 0.1 mL of water from the sample.
Molluscs were analyzed to determine the MPN of E. coli (series of three tubes and three dilutions) and the presence of Salmonella spp., V. parahaemolyticus and V. cholera based on Pouch & Ito (2001). A total of 25 g of flesh and intravalvular liquid was used with serial dilutions (10-1 to 10-3) inoculated in triplicate using tubes with Lauryl Tryptose Broth.
The confirmatory phase for both samples was performed in Brilliant Green Bile Broth (OXOID®) for total coliforms and EC Broth (OXOID®) for E. coli. E. coli was isolated using MacConkey Agar (OXOID®) and Levine Agar (EMB) (OXOID®) and identified through IMViC (OXOID®) biochemical tests and miniaturized API® 20 E tests (Biomerieux®).
The presumptive determination of Salmonella spp. was made using Buffered Peptone Water (BD®) for pre-enrichment homogenization followed by enrichment with Rappaport-Vassiliadis Broth (BD®) and Tetrathionate Broth (LIOFILCHEM®). Typical colonies isolated on XLD Agar (BD®) were verified using biochemical tests IMViC, Triple Sugar Iron Agar (BD®) and Urea Agar (OXOID®).
On the other hand, 25 g of flesh and intravalvular liquid were homogenized in 225 mL of Buffered Peptone Water with 3% NaCl and 25 g of flesh and intravalvular liquid in 225 mL of Alkaline Peptone Water (OXOID®) for the enrichment of V. parahaemolyticus and V. cholerae, respectively. Isolation was conducted in TCBS Agar (BD®) and identification was achieved using biochemical tests including Triple Sugar Iron Agar, Dextrose Oxidation/Fermentation (BD®), Decarboxylase Medium Base (Arginine, Ornithine) (BD®), Indole, MR-VP Broth (BD®), Gelatin (BD®) and miniaturized API® 20 NE tests (Biomerieux®).
Results were graphed using statistical programming language R version 2.15.0 (R Core Team, 2012).
RESULTS
Water used in the depuration treatment presented minimal values of E. coli (<2 and 2 MPN/100 mL) in the initial samples (Table 1), although in treatments B and D water was not previously disinfected. These results indicate that the water catchment area meets, in this case, the microbiological quality specifications that are required for these procedures (Lee et al. 2010; Oliveira et al. 2011).
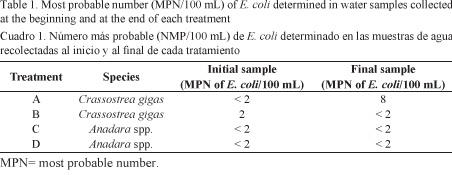
In the water of treatment A, the MPN of E. coli increased at the end of the depuration process (8 MPN/100 mL), while treatment B shows a decrease (<2 MPN of E. coli/100 mL). Values of E. coli in treatments C and D were below 2 MPN/100 mL throughout the entire process (Table 1).
Initials samples of C. gigas and Anadara spp. showed higher concentrations of E. coli (Fig. 2) than those recommended by the FAO for direct human consumption (Lee et al. 2010). In other words, all treatments showed values higher than 230 MPN of E. coli/100 g of flesh and intravalvular liquid. Treatment A recorded the highest concentration of E. coli (4.6x104 MPN/100 g) (Fig. 2). For this reason, depuration of these organisms is necessary prior to their commercialization and consumption.
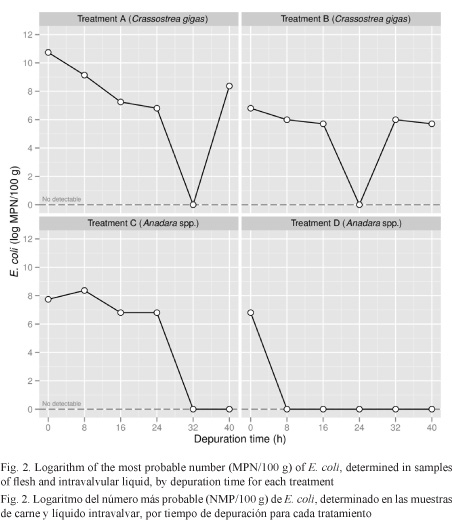
Results obtained confirm that the system used reduced in the first 24 h between 98% and 100% of the initial contamination by E. coli in oyster C. gigas and between 79% and 100% of contamination in mangrove cockles Anadara spp. (Fig. 3).
Non-detectable values of E. coli for C. gigas in treatments A and B were reached at h 32 and h 24, respectively. After this period, the MPN of E. coli in flesh increased again to 4.3x103 and 4x102 in treatments A and B, respectively (Fig. 2). The 100% depuration in Anadara spp. was obtained at h 32 in treatment C and at h 8 in treatment D (Fig. 2 and 3).
None of the analyzed samples showed the presence of Salmonella spp., V. parahaemolyticus or V. cholerae. Mortality of molluscs was not observed in any of the treatments.
DISCUSSION
High concentrations of E. coli determined on initial samples analyzed confirm the potential public health risk posed by these products. This risk is increased due to the fact that oysters and cockles are usually consumed immediately after being harvested without any previous treatment such as depuration, refrigeration, or cooking (Lee et al. 2010; Oliveira et al. 2011).
The presence of E. coli in C. gigas and Anadara spp. can be related to the ability of bivalve molluscs to accumulate pollutants found in coastal environments (Ho & Tam, 2000; Clark, 2001) or the lack of proper handling and processing (Oliveira et al. 2011).
Depuration is one of the most commonly used treatments to decrease bacterial contamination in bivalve molluscs (Oliveira et al. 2011). The effectiveness of this process depends on the design and management of the system, as well as factors such as the species (Lee et al. 2010), physiological state (Cusson et al. 2005), spawning periods (López et al. 2005), water temperature and salinity (Love et al. 2010), initial concentration and type of contamination found in these organisms, and regional geographical and seasonal characteristics (Oliveira et al. 2011).
The results of this study prove the effectiveness of the small scale E. coli depuration system used in C. gigas and Anadara spp. In all treatments, analyzed molluscs reached, according to Lee et al. (2010) and Oliveira et al. (2011), optimal values of E. coli for human consumption. This is the first report to describe the depuration of C. gigas in Costa Rica and the first report of UV depuration for bacterial indicators of fecal contamination in Anadara spp.
The recolonization of E. coli in the system (treatment A) and C. gigas (treatments A and B), after reaching a 100% depuration level, could be explained by the resuspension of sediment material in the system. As stated by Lee et al. (2010), bacterial pathogens can survive during the depuration process in the filamentous feces of bivalve molluscs, producing recontamination if the excreted fecal material is ingested by the organisms. This recontamination could also be due to the high number of oysters (C. gigas) used in treatments A and B; which could be an indicator, for this species, of the load capacity of the system used. In treatments C and D applied to Anadara spp. no recontamination by E. coli was observed possibly due to the much smaller amount of molluscs used. The excessive volume of organisms in the depuration ponds reduces purification efficiency and may increase the microbiological burden due to recontamination, the obstruction in the flow of water, and the accumulation of final metabolic products (Lee et al. 2010; Oliveira et al. 2011).
It should be kept in mind that depuration is not effective for bivalve molluscs from areas with high levels of anthropogenic pollution and does not reduce contamination caused by hydrocarbons, heavy metals, pesticides, or biotoxins (Lee et al. 2010). Likewise, human enteric viruses cannot be eliminated through conventional depuration (Nappier et al. 2008) because they can survive longer than bacteria indicating fecal contamination (Love et al. 2010; de Abreu et al. 2012).
Vibrio species are also not eliminated through depuration methods used for E. coli (Croci et al. 2002) and currently represent one of the main natural contaminants of the marine environment (Oliveira et al. 2011). This study did not detect the presence of V. parahaemolyticus and V. cholerae in any of the samples.
The best method to safely produce bivalve molluscs is harvesting and extracting them in areas free from external pollution. The production of bivalve molluscs in areas with relatively low pollution levels followed by a depuration process guarantees a risk level as low as the one reached through cooking (Lee et al. 2010).
Although depuration increases production costs (Cusson et al. 2005), it also promotes the development of economic activities aimed towards a better use of bivalve molluscs, improving the quality and innocuousness of the product and increasing commercial value for producers (de Abreu et al. 2007). Additionally, UV irradiation offers the advantage of requiring less water than a continuous flow system (de Abreu et al. 2007), as well as a lower investment and smaller operational costs in comparison to other disinfection methods. It is also highly effective and does not cause any risks for personnel or residual effects such as toxic products or chemicals used on the water or the molluscs (Lee et al. 2010).
This research preliminarily proves the effectiveness of the small scale depuration system design for E. coli in C. gigas and Anadara spp. in Costa Rica, offering a low cost alternative to eliminate bacterial pollutants after harvesting the studied molluscs, as well as increasing the value of oysters and cockles for producers. In addition, it serves as a basis for the implementation of more detailed studies that determine the effect of parameters such as density of organisms, flow, temperature, salinity, dissolved oxygen and physiological state of molluscs in order to establish the optimal conditions for purification of microbial contaminants in C. gigas and Anadara spp. It also offers an option to reduce potential risks to public health resulting from the consumption of these marine products.
In order to ensure a proper sanitary control of bivalve molluscs in Costa Rica, pollution regulations, classification and monitoring should be implemented in the extraction and harvesting areas. Good bivalve mollusc handling and processing practices as well as a proper depuration and preservation should also be implemented to prevent the evaluated risks.
ACKNOWLEDGMENTS
We would like to thank Estación Nacional de Ciencias Marino Costeras (National Coastal Marine Science Station) of Universidad Nacional (ECMAR-UNA) for allowing us to use their infrastructure and Asociación de Mujeres Trabajadoras del Marisco de Chomes (Chomes Women’s Seafood Workers’ Association), Asociación de Mujeres de Punta Morales (Punta Morales Women’s Association) and Asociación de Proyectos Pesqueros de Costa Pájaros (Costa de Pájaro Fisheries Projects’ Association) for providing us with the samples.
This research was financed by Consejo Nacional de Rectores (CONARE) (the National Provost Council), as part of the Project entitled “Incremento en la competitividad de las PYMES del Pacífico Central mediante un plan de fortalecimiento interuniversitario regional” (“Increasing competitiveness of SMEs in the Central Pacific by an inter-university regional strengthening plan”), and Ley de Pesca y Acuicultura (Fisheries and Aquaculture Law) from the Government of Costa Rica, and by the Project entitled “Composición nutricional y efecto de las alteraciones bioquímicas, microbiológicas y ambientales en la calidad y la frescura de algunas especies de interés comercial capturadas en el Golfo de Nicoya” (“Nutritional composition and effect of environmental, microbiological and biochemical alterations in the quality and freshness of some commercial species caught in the Gulf of Nicoya”).

Preliminary assessment of small scale bacterial depuration of Crassostrea gigas and Anadara spp., Gulf of Nicoya, Costa Rica por Revista Ciencias Marinas y Costeras se distribuye bajo una Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Costa Rica License.
Basada en una obra en http://www.revistas.una.ac.cr/index.php/revmar.
Permisos que vayan más allá de lo cubierto por esta licencia pueden encontrarse en revmar@una.cr.
REFERENCES
Clark, C. (2001). Marine pollution. New York, USA: Oxford University Press.
Clesceri, L. S., Greenberg, A. E. & Trussell, R. R. (1989). Standard methods for the examination of water and wastewater. Washington, USA: American Public Health Association, American Water Works Association, Water Pollution Control Federation.
Croci, L., Suffredini, E., Cozzi, L. & Toti, L. (2002). Effects of depuration of molluscs experimentally contaminated with Escherichia coli, Vibrio cholerae 01 and Vibrio parahaemolyticus. J. Appl. Microbiol., 92, 460-465.
Cusson, M., Tremblay, R., Daigle, G. & Roussy, M. (2005). Modeling the depuration potential of blue mussels (Mytilus spp.) in response to thermal shock. Aquaculture, 250, 183-193.
de Abreu, A., Albarnaz, J. D., Moresco, V., Poli, C. R., Teixeira, A. L., Oliveira, C. M. & Monte, C. R. (2007). Depuration dynamics of oysters (Crassostrea gigas) artificially contaminated by Salmonella enterica serovar Typhimurium. Mar. Environ. Res., 63(5), 479-489.
de Abreu, A., Rigotto, C., Moresco, V., Kleemann, C. R., Teixeira, A. L., Poli, C. R., Oliveira, C. M. & Monte, C. R. (2012). The depuration dynamics of oysters (Crassostrea gigas) artificially contaminated with hepatitis A virus and human adenovirus. Mem. Inst. Oswaldo Cruz, 107(1), 11-17.
Ho, B. S. W. & Tam, T.-Y. (2000). Natural depuration of shellfish for human consumption: a note of caution. Water Res., 34(4), 1401-1406.
Lee, R., Lovatelli, A. & Ababouch, L. (2010). Depuración de bivalvos: aspectos fundamentales y prácticos. FAO Documento Técnico de Pesca No. 511. Rome, Italy: FAO.
López, C., Darriba, S., Miranda, M. & Álvarez, C. (2005). Depuración de la navaja Ensis arcuatus (Jeffreys, 1865) y el longueirón Ensis siliquia (L., 1758) (Solenacea). Bol. Inst. Esp. Oceanogr., 21(1-4), 311-315.
Love, D. C., Lovelace, G. L. & Sobsey, M. D. (2010). Removal of Escherichia coli, Enterococcus fecalis, coliphage MS2, poliovirus, and hepatitis A virus from oysters (Crassostrea virginica) and hard shell clams (Mercinaria mercinaria) by depuration. Int. J. Food Microbiol., 143, 211-217.
MacKenzie, C. L. (2001). The fisheries for mangrove cockles, Anadara spp., from Mexico to Peru, with descriptions of their habitats and biology, the fishermen’s lives, and the effects of shrimp farming. Mar. Fish. Rev., 63(1), 1-39.
Nappier, S. P., Graczyk, T. K. & Schwab, K. J. (2008). Bioaccumulation, retention and depuration of enteric viruses by Crassostrea virginica and Crassostrea ariakensis oysters. Appl. Environ. Microbiol., 74(22), 6825-6831.
Oberbeckmann, S., Fuchs, B. M., Meiners, M., Wichels, A., Wiltshire, K. H. & Gerdts, G. (2012). Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb. Ecol., 63, 543-551.
Oliveira, J., Cunha, A., Castilho, F., Romalde, J. L. & Pereira, M. J. (2011). Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives - A mini-review. Food Control, 22(6), 805-816.
Pouch, F. & Ito, K. (2001). Compendium of methods for the microbiological examination of foods. Washington, USA: American Public Health Association.
R Core Team. (2012). R: A language and environment for statistical computing. Version 2.15.0. Vienna, Austria: R Foundation for Statistical Computing.
Silva, A. M. & Bonilla, R. (2001). Abundancia y morfometría de Anadara tuberculosa y A. similis (Mollusca: Bivalvia) en el manglar de Purruja, Golfo Dulce, Costa Rica. Rev. Biol. Trop., 49(Supl. 2), 315-320.