Rev. Mar. Cost. ISSN 1659-455X. Vol. 5: 119-134, Diciembre 2013.
Study on the etiology of fibropapillomatosis of olive ridley sea turtles (Lepidochelys olivacea) nesting in the National Wildlife Refuge at Ostional, Guanacaste, Costa Rica
Estudio de la etiología de la fibropapilomatosis de la tortuga lora (Lepidochelys olivacea) que anida en el Refugio Nacional de Vida Silvestre de Ostional, Guanacaste, Costa Rica
Laura Brenes Chaves1*, Alexis Berrocal1, Ana I. Meneses¹, Carlos Jiménez Sánchez1 & Carlos M. Orrego Vásquez2
1 Escuela de Medicina Veterinaria, Universidad Nacional de Costa Rica. sobrecha@yahoo.com*, histopatovet@gmail.com, anagmeneses@gmail.com, carlos.jimenez.sanchez@una.cr
2 Área de Conservación Tempisque, Sistema Nacional de Áreas de Conservación. corregovasquez@gmail.com
Recibido 30 IV 2013
Aceptado 05 XI 2013
ABSTRACT
Sea turtle fibropapillomatosis is an emerging disease characterized by a proliferation of cutaneous papillomas, fibromas, and fibropapillomas and occasional visceral fibromas. This paper aims to contribute to the etiology of fibropapillomatosis in olive ridley sea turtles (Lepidochelys olivacea) nesting in Ostional National Wildlife Refuge. Twenty-six olive ridley turtles with cutaneous fibropapilloma were sampled and 24 healthy olive ridley turtles served as controls. Biopsies were taken of the cutaneous tumors in sick sea turtles, as well as skin biopsies from control subjects, and blood samples were collected from all turtles. Tumorous samples and skin samples were microscopically analyzed in order to differentiate the histological factors resulting from the disease pathogenesis, where the main histological findings were papillary epidermal hyperplasia, orthokeratotic hyperkeratosis, spirorchid-like eggs in the dermis, and eosinophilic cytoplasmic inclusion. Hematology and blood chemistry studies were conducted on blood samples, and MCHC, heterophils, lymphocytes, monocytes, AST, total protein, albumin and globulin values were significantly different between healthy turtles and turtles with tumors. A PCR test was also conducted in the samples to determine the presence of herpesvirus and papillomavirus as possible etiologic agents, where the papilomavirus was absent in all the samples, while the herpesvirus was present in 69.23% of the tumors, this being the most probable etiological agent of fibropapillomatosis.
Keywords: Lepidochelys olivacea, fibropapilloma, PCR, herpesvirus.
RESUMEN
La fibropapilomatosis de la tortuga marina es una enfermedad emergente caracterizada por múltiples papilomas, fibromas y fibropapilomas cutáneos, así como ocasionales fibromas viscerales. El presente trabajo tiene como objetivo contribuir a la etiología de la fibropapilomatosis en la tortuga lora (Lepidochelys olivacea) que anida en el Refugio Nacional de Vida Silvestre Ostional. Se muestrearon 26 tortugas lora con fibropapilomas cutáneos y 24 tortugas lora sanas que sirvieron de control. Se tomaron biopsias excisionales de los tumores cutáneos de las tortugas enfermas y biopsias de piel de las tortugas control, además se recolectaron muestras de sangre de todas las tortugas. Las muestras tumorales y de piel se analizaron microscópicamente para diferenciar los factores histológicos que resultan de la patogénesis de la enfermedad, donde los hallazgos histopatológicos principales en los fibropapilomas fueron: crecimiento papiliforme, hiperqueratosis ortoqueratótica, huevos de parásitos similares a espiróquidos en la capa dérmica e inclusión eosinofílica citoplasmática. A las muestras de sangre se les realizó análisis hematológico y de química sanguínea, donde los valores de CHCM, heterófilos, linfocitos, monocitos, AST, proteínas totales, albúmina y globulinas resultaron significativamente diferentes entre las tortugas sanas y las tortugas con tumores. Se realizó PCR a las muestras para determinar la presencia de genoma de herpesvirus y papilomavirus como posibles agentes etiológicos, donde el papilomavirus estuvo ausente en la totalidad de las muestras, mientras el virus Herpes se presentó en el 69.23% de los tumores posicionándose como el posible agente etiológico de la enfermedad.
Palabras claves: Lepidochelys olivacea, fibropapiloma, PCR, herpesvirus.
INTRODUCTION
Fibropapillomatosis is an emerging disease described mainly in green turtles (Chelonia mydas), whose first reports were conducted at the New York Aquarium in 1938 (Smith & Coates, 1938) and Cape Sable, Florida (Lucke, 1938). Subsequently, it was reported in 1958 in green turtles in Hawaii (Jacobson et al. 1989), in 1980 in green turtles in captivity on Grand Cayman and the British East Indies, in 1982 in the India River Lagoon in Florida (Herbst, 1994), and in 1996 in Monroe County, Florida (Orós et al. 1999).
Lesions similar to the green sea turtle fibropapillomatosis were observed in the loggerhead turtle (Caretta caretta) from Florida and Australia (Balasz & Pooley, 1991; Herbst et al. 1998); the flatback turtle (Natator depressus) in Australia (Herbst, 1994), and the olive ridley turtle (Lepidochelys olivacea) on the Pacific coast of Costa Rica (Chaves et al. 1998; Orrego & Morales, 2002).
In 1987 the first case of fibropapillomatosis was reported in Costa Rica, in an olive ridley turtle in the Ostional National Wildlife Refuge (Refugio Nacional de Vida Silvestre Ostional-RNVSO), where the animal showed signs of an advanced disease with 30 mm diameter tumors (Orrego & Morales, 2002). Subsequently, the number of turtles with tumors increased in Ostional as well as the size of the lesions (Orrego & Work, 2004). Most of the lesions were located on the neck, around the eyes, the mouth and the carapace dorsal edges and between the scutes (Chaves et al. 1998).
In Ostional, Aguirre et al. (1999) conducted a study between July and September, 1997, in which 50 tumor biopsies were collected from 25 affected animals, whose masses were macroscopically 25 mm in diameter, had grayish-white coloration, and were located on the skin of the neck and flippers. Histologically, 42 of the 50 samples were classified as fibropapillomas, of which 20 were in a state of regression.
Fibropapillomatosis is currently considered a disease that has spread worldwide, with a prevalence range of 0% to 92% in some areas (Herbst, 1994). The most affected ages are juveniles, subadults, and adults. This neoplasm has been associated with a herpesvirus (Herbst, 1994; Quakenbush et al. 1998; Lackovich et al. 1999; Orós et al. 1999; Lu et al. 2000; Quakenbush et al. 2001; Coberly et al. 2002; Greenblatt et al. 2005; Ene et al. 2005), but the role of this agent as a cause of the disease has not yet been completely clarified and it is possible that other factors, such as the quality of the environment, play an important role (dos Santos et al. 2010).
The main clinical signs include multiple cutaneous fibroepithelial tumors anywhere with soft and hard tissue (Jacobson et al. 1989). They are common in the orbital and periorbital regions (Brooks et al. 1994) and in the oral cavity (Herbst, 1994). Turtles affected by the tumor exhibited signs of wasting, weakness, depression, and anemia. Given the nature of this pathology, turtles may also exhibit metastasis with multiple tumors of fibrous consistency in lungs, liver, kidneys and gastrointestinal tract, causing problems such as floating, bowel obstruction, renal failure, and necrosis by the compression of the affected tissues (Herbst, 1994; Herbst et al. 2001). The main objective of the present research was to contribute, through pathological and histopathological, virological, molecular, and hematology analysis, to the knowledge on etiology and the impact of fibropapillomatosis on olive ridley turtles nesting in RNVSO, Guanacaste, Costa Rica.
MATERIALS AND METHODS
Study site and animals
The research was conducted between June 2004 and February 2005, in Playa Ostional (coordinates 10° 00’ 00” N, 86° 45’ 50” W), which is 3.9 km long and is located in Santa Cruz, Guanacaste, within the Ostional National Wildlife Refuge.
The species studied was the olive ridley turtle due to its importance at this nesting site, which is one of the most important sites worldwide (Ballestero et al. 1998; Araya et al. 2002; Valverde et al. 2012).
For the identification of healthy and tumorous turtles, day and night walks were taken during the time of arrival or mass nesting, covering the entire length of the beach and externally reviewing turtles that came to nest in order to determine the presence of possible tumors and, in the case of control turtles, inspecting those that had no wounds or visible growths. Turtles included in the study were given a physical examination, and the number of tumors, as well as their distribution and size was determined for sick subjects. All turtles in the study were marked with Inconel metallic plates, style 1005-681, according to the protocol used by Eckert & Begge (2006), before being released into the sea.
Samples for pathology and histopathology
Tumors were collected through excisional biopsies, and skin biopsies were performed in control turtles of about 2 cm long and half a centimeter thick in flippers, neck or the axillary region using local anaesthetic (Davidson et al. 1998). The collected tissue was fixed in 10% formalin (Eckert et al. 2000) and, subsequently, samples were analyzed at the Pathology Lab of the School of Veterinary Medicine at Universidad Nacional (UNA). Tissues were embedded in paraffin using routine methods. Samples were sectioned 5-6 micrometers thick and stained with Hematoxylin and Eosin (H&E).
Samples for virological tests
Tissue collected for viral examination was placed in a sterile vial containing viral transport medium (Eckert et al. 2000) and was later taken in a cooler to the Virology Lab at the School of Veterinary Medicine (Universidad Nacional) and stored at -70˚C until it was analyzed.
DNA extraction
The Wizard Genomic DNA Purification Kit (PROMEGA, USA) was used to extract deoxyribonucleic acid (DNA) from tissues according to the manufacturer’s specifications. DNA obtained was stored at -20˚C until it was analyzed.
Polymerase chain reaction (PCR), primers, and PCR protocol for herpesvirus and papillomavirus
PCR was performed for herpesvirus and papillomavirus. Primers FHV (5’-AGCATCATCCAGGCCCACAATCT-3’) and HV (5’-CGGCCAGTTC-CGGCGCGTCGACCA-3’), described by Lu et al. (2000), were used for the detection of the viral polymerase gene of herpesvirus, while the primers described by Forslund et al. (1999), FAP59 (5’TAACWGTIGGICAYCCWTATT3’) and FAP64 (5’CCWATATCWVHCATITCICCA-TC3’), were used for the detection of the papillomavirus L1 gene. Reactions were performed in a volume of 25µl using an Applied Biosystems thermal cycler (2 720) and 2X PCR Master Mix reagents (Fermentas, cat. K0179). In both cases, after incubation at 94°C for 5 min, DNA amplification was performed by 40 cycles of 94°C (60 sec.), 50ºC (60 sec.) and 72ºC (60 sec.) and a final extension at 72°C for 7 minutes. Amplicons were observed with an ultraviolet light transilluminator after electrophoresis on 2% agarose gel and staining with ethidium bromide. Expected products of the herpesvirus polymerase gene and the papillomavirus L1 gene are 445bp and 478bp, respectively. A 50-2 000 bp ladder (Sigma) was used as molecular weight markers.
Statistical analysis of PCR results
The results of both healthy and sick groups were grouped in 2x2 tables and analyzed using the McNemar’s Test with the GraphPad Software (2013).
Samples for blood tests
Blood samples were collected from dorsal cervical sinus of selected healthy and sick animals (Beynon & Cooper, 1999; Murray, 2000), and were deposited in tubes with heparin and tubes without anticoagulant. Samples were sent in a cooler at 4°C to the Clinical Analysis Lab of the School of Veterinary Medicine at UNA, where complete blood count and serum chemistry tests were performed including phosphorus, calcium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose, urea, urea nitrogen, creatinine, total protein, albumin, globulin and albumin-globulin ratio (Eckert et al. 2000).
Statistical analysis
The statistical analysis of blood test results was performed through hypothesis testing using the Student’s T test.
RESULTS
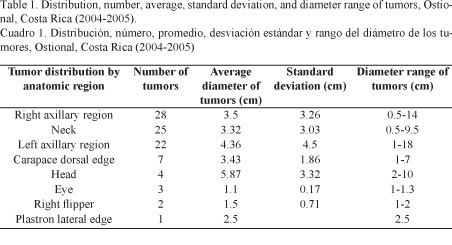
A total of 2 054 adult female olive ridley turtles were monitored, 26 of which had tumor masses compatible with fibropapilloma (Fig. 1) and 24 served as control. From the 26 turtles presenting tumors, 6 had a single lesion, whereas the remaining 20 showed multiple lesions up to a maximum of 12 tumors in one single subject. The tumors’ anatomical location and dimensions can be seen in Table 1. Some masses were warty and had soft consistency with pinkish-white coloration. Bigger and more developed masses had a cauliflower-like appearance and hard consistency, were grayish green, sessile or pedunculated stalked and frequently ulcerated.
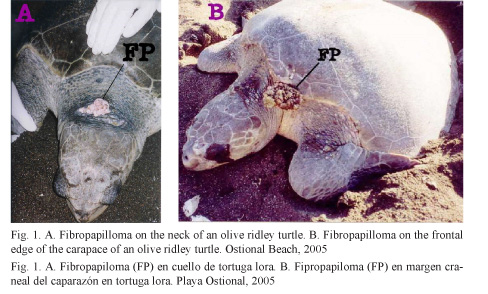
Histologically, normal olive ridley turtle skin epidermis consists of a stratum corneum comprised of two to four layers of keratin and a stratum spinosum comprised of four to seven cells thick stratified squamous epithelium. The dermis is comprised of stratum papillare with thin layers films of collagen tissue, small mononuclear cells, blood vessels, and chromatophores extending to the adjacent reticular layer. The reticular layer consists of large layers of collagen tissue, fibroblasts, and blood vessels with perivascular mononuclear cells (Fig. 2).
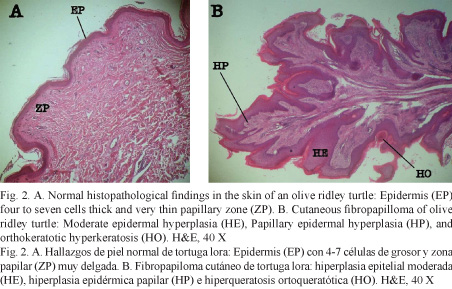
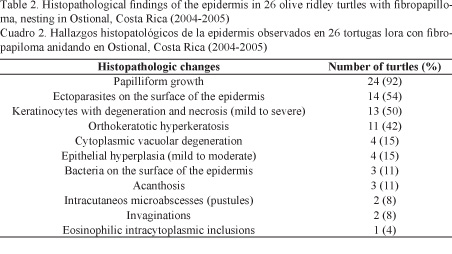
The most important histopathological findings detected in the epidermis (Table 2) and dermis (Table 3) of tumors can be seen in Fig. 2.

Analyses of DNA extracted from tumors and biopsies of healthy skin by PCR allowed identifying the herpesvirus polymerase gene in 69.23% of the tumor lesions and in only 4.16% of the biopsies of healthy skin. On the other hand, the L1 capside protein gene of papillomavirus was not detected in any of the samples. The statistical analysis, McNemar’s Test, applied to the herpesvirus results was highly significant, P < 0.0001, OR = 5.667, with 95% confidence intervals of 2.763-13.093.
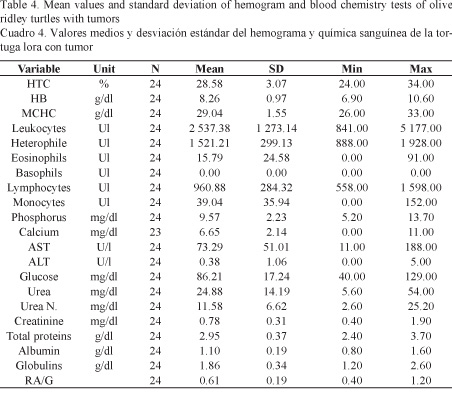
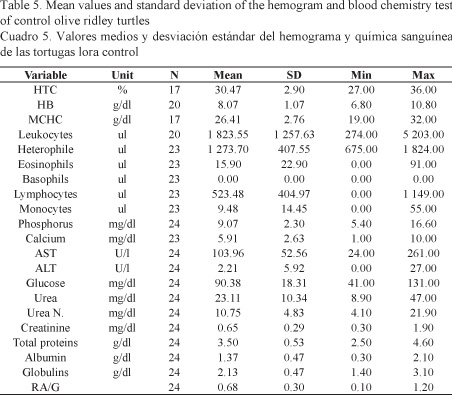
The results of the hemogram and blood chemistry analyses obtained for each group of turtles are descriptively summarized in Tables 4 and 5. The statistical analysis, i.e. hypothesis testing using the Student’s T test, pointed out significant differences for the following values: MCHC (P = 0.0017), Heterophile (P = 0.0216), Lymphocytes (P = 0.0001), Monocytes (P = 0.0008), AST (P = 0.0460), total protein (P = 0.0002), Albumin (P = 0.0125), and Globulin (P = 0.0286).
DISCUSSION
This is the first study conducted in olive ridley turtles in Costa Rica involving subjects suffering from fibropapilloma and healthy animals used as control, combining three research areas: histopathology, virology, and blood chemistry and hematology analysis.
Histopathological results of samples of proliferative skin lesions are consistent with what has been reported previously for fibropapilloma in green turtles and olive ridley turtles (Smith & Coates, 1938; Jacobson et al. 1989; Brooks et al. 1994; Herbst, 1994; Herbst et al. 1995; Herbst et al. 1998; Aguirre et al. 1999; Herbst et al. 1999; Vale & Bracho, 2000; Gámez et al. 2009; Rodenbusch et al. 2012). Fibropapilloma develops in sea turtles similarly as it does in mammals. The tumor begins with a proliferation of fibroblasts in the superficial dermis, followed by an epidermis proliferation with acanthosis and orthokeratosis. As the nodules enlarge, the epidermis becomes verrucous. Degenerative and lithic changes develop subsequently in the stratum spinosum and stratum basale, ending with the separation of the dermis-epidermis and the formation of vesicles and subsequent ulceration. The continuous proliferation of fibroblasts widens the epidermis, resulting in a soft and firm mass (Herbst et al. 1999; Greenblatt et al. 2005; Rodenbusch et al. 2012).
The most important histopathological findings associated to the etiology of fibropapillomatosis are eosinophilic cytoplasmic inclusions and spirorchid-like parasitic eggs. Spirorchid eggs were first reported by Smith & Coates (1938). Balazs (1986) later found them again in fibropapillomas, which suggests a strong relationship of these parasites as a possible etiologic agent of the disease. However, Herbst (1994) was not able to experimentally induce fibropapilloma to turtles through the injection of spirorchid material and Herbst et al. (1998) could not find any relationship between the exposure to spirorchids and fibropapilloma; therefore, they were dismissed as causative agents of the disease. In Costa Rica, spirorchid eggs have been reported by Santoro et al. (2007) in the Caribbean, as the cause for granulomatous reactions, vasculitis, thrombi and arteritis in the large vessels of green turtles. Therefore, the presence of spirorchid eggs in the vessels of the tumor dermis is possible due to the inflammatory and angiogenesis processes taking place in the fipropapillomatosis, moving the eggs by the circulatory system from the large blood vessels and the heart to the tumor dermal surface.
The presence of an eosinophilic cytoplasmic inclusion in the tumor of an olive ridley turtle in this study is consistent with the appearance of eosinophilic inclusions in other studies (Jacobson et al. 1989; Herbst et al. 1995; Vale & Bracho, 2000). In a study on experimental transmission conducted by Herbst et al. (1999), experimentally induced tumors were compared to spontaneous tumors, where the former, which were the youngest samples, had the highest frequency of inclusions. If the virus production and its dissemination are transitory events present early in the progression of the disease, evidence of an active infection would decrease with age, which would explain why only one turtle in this study presented intracytoplasmic eosinophilic inclusions since only adult turtles were sampled.
In the study conducted by Herbst et al. (1998) on the serological association between fibropapilloma and infection caused by herpes, a strong relationship was established between the conversion of reagents to herpesvirus antibodies and the development of experimentally induced tumors. In addition, it was determined that it was also possible that the tumor was related to a physiological environment favoring the reactivation of a latent infection by herpesvirus, along with a resurgence of anti-herpesvirus antibody titers. Consequently, it could not be concluded that herpesvirus was the definitive etiologic agent of fibropapilloma.
PCR analyses detected the herpesvirus polymerase gene in 69.23% of tumor lesions and in only 4.16% of the biopsies of healthy skin. Statistically, a close relationship was established between tumor lesions and the herpesvirus genome. This data is consistent with a study conducted by Lu et al. (2000) in green turtles from Hawaii, where samples of cutaneous tumors analyzed by primary PCR showed the presence of herpesvirus in 87% of the tumors and the absence of the virus in control samples. Another study conducted by Quackenbush et al. (1998) in green turtles from Hawaii and Florida and olive ridley turtles from Costa Rica determined by nested PCR that 100% of the tumors had herpesvirus. Ene et al. (2005) established the presence of herpesvirus associated with cheloniidae fibropapilloma (C-FP-HV) in green turtles from Florida, including four variants A, B, C, D, where variants A and C were presented in different species of sea turtles such as L. kempii and L. olivacea. In summary, the evidence found in this study and other research worldwide (Quackenbush et al. 1998; Lackovich et al. 1999; Lu et al. 2000; Quackenbush et al. 2001; Coberley et al. 2002; Ene et al. 2005; Greenblatt et al. 2005; Rodenbusch et al. 2012) suggest the involvement of herpesvirus in the etiology of the marine turtle fibropapillomatosis.
PCR results determined that none of the turtles with tumors or control turtles were positive to papillomavirus. In many vertebrate species, the papillomavirus causes papillomas, fibroids, and fibropapillomas, and hyperplastic lesions can be observed in side-necked turtles (Acanthochelys pallidipectoris) in Bolivia. Similarly, herpesvirus has also been found in green lizard papillomas (Herbst et al. 1995), which was the reason why it was important to include it in this study. However, like other preliminary studies aimed at relating papillomavirus antigens with fibropapilloma, results were negative (Herbst, 1994; Jacobson et al. 1989).
According to the hematology and blood chemistry studies between healthy turtles and turtles with tumors, significant differences were found only between 4 hematological values: MCHC, heterophils, lymphocytes and monocytes. In the case of the MCHC, the mean value obtained was similar to the one reported by Santoro & Meneses (2007) for healthy turtles in Ostional. This same value was significantly lower in turtles with tumors compared to healthy turtles maybe due to an iron deficiency (hypochromia) or an inappropriate response of the organism to a chronic disease such as the one caused by the presence of fibropapilloma (Meneses et al. 1993; Aguirre et al. 1995; Work & Balazs, 1999).
The value of heterophils was significantly lower in turtles with tumors than in healthy turtles. This data differs from other studies on green turtles, in which heterophil was actually found (Aguirre et al. 1995; Work & Balazs, 1999). Heteropenia could be caused by chronic inflammatory processes due to a poor response of the immune system (Meneses et al. 1993).
Turtles with a tumor were determined to have lymphopenia, which has also been found in other studies on green turtles with fibropapilloma. This may be due to a suppression of the immune system associated with the initial stages of viral infections (Meneses et al. 1993; Aguirre et al. 1995; Work & Balazs, 1999).
Turtles with tumors had less monocytes than healthy turtles. According to Work & Balazs (1999) green turtles with fibropapilloma have monocytosis as a response to a chronic infectious process or any other immunogenic stimulation; however, according to Aguirre et al. (1995) the absence of monocytes can be a normal finding in turtles with tumors.
Differences were found by comparing the values obtained in this study from the blood chemistry test of healthy turtles with the values obtained by Santoro & Meneses (2007) regarding ALT, glucose, urea nitrogen and total protein. These differences could be related to the physiological state of the turtles at the time of sampling. In the case of Santoro & Meneses (2007), samples were obtained from reproductively active females copulating in the sea, while the observation in the present study was from females nesting in the sand. It has been suggested that sea turtles do not feed actively during the breeding season; consequently, their energy reserves are limited and will vary according to the time span that they have not been eating (Owen, 1980).
Significant differences were found between healthy turtles and turtles with tumors in four values: AST, total protein, albumin, and globulin, whose changes may also be related to the physiological state of the turtles or may be indicators of chronic or pathological conditions (Meneses et al. 1993).
In the case of green turtles in other investigations, turtles with advanced fibropapilloma had hypoproteinemia, hypoalbuminemia, and hypoglobulinemia, which is a common finding in chronic and debilitating diseases (Aguirre et al. 1995; Aguirre & Balazs, 2000). However, these results differ from the calculated values, since they increased slightly in turtles with tumors. The increase in total proteins is directly related to the increase in globulins. In turn, increased globulins may result from the increase of total acute phase proteins, which also increase in inflammatory processes as the ones represented in the early development stages of the tumor (Meneses et al. 1993).
The AST increase in turtles with fibropapilloma compared to control turtles coincides with studies on green turtles with this type of tumor, where the increase in this enzyme is indicative of chronic stress and muscle damage (Aguirre et al. 1995; Aguirre & Balazs, 2000; Meneses et al. 1993).
CONCLUSIONS
Fibropapillomatosis is a disease that has been on the rise both in terms of the geographical location and the species that have it. In Costa Rica, since it was reported for the first time in Ostional, tumors have been observed with greater frequency and anatomical distribution, mainly located in the soft tissue in the axillary region and neck, ranging from 0.5 to 18 cm in diameter. Removed tumors were single or multiple masses that varied from a wart-like appearance of soft consistency and pinkish-white coloration to large masses of cauliflower-like appearance, hard consistency and grayish-green coloration.
In the histopathological area, the proliferative skin lesions described are consistent with those previously reported for fibropapilloma in green turtles and olive ridley turtles. The main histopathological findings in fibropapillomas of the studied turtles were papilliform growth, spirorchid-like parasite eggs in the dermal layer, degeneration and necrosis of keratinocytes, orthokeratotic hyperkeratosis, and cytoplasmic eosinophilic inclusion.
Hematological and blood chemistry values reported by Santoro & Meneses (2007) were similar to the values obtained, except for ALT, glucose, urea nitrogen, and total protein, which differed possibly due to the physiological reproductive state of the turtles at the time of sampling. In addition, the values for MCHC, heterophile, lymphocytes, monocytes, AST, total protein, albumin and globulin are significantly different between healthy turtles and turtles with tumors due to an inadequate response of the organism to a chronic disease such as the one caused by the presence of fibropapilloma.
On one hand, PCR analyses allowed the identification of the herpesvirus polymerase gene in 69.23% of tumor lesions and in only 4.16% of the biopsies of healthy skin. On the other hand, the L1 capside protein gene of papillomavirus was not detected in any of the samples. Therefore, it is concluded that the etiologic agent of the disease may be herpesvirus; however, further research is recommended on the transmission of the disease to test its etiology. Since this virus can be persistent, its high presence in this research can also be due to the fact that the tumor may have a physiological environment favoring the reactivation of infection by latent herpes.
In the case of Ostional, which is one of the most important sites of mass nesting for olive ridley turtles, the repercussions that the increased prevalence of the disease could have are important not only because it affects a species vulnerable to extinction but because it threatens the ecological balance holding nesting populations in this refuge.

Study on the etiology of fibropapillomatosis of olive ridley sea turtles (Lepidochelys olivacea) nesting in the National Wildlife Refuge at Ostional, Guanacaste, Costa Rica por Revista Ciencias Marinas y Costeras se distribuye bajo una Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Costa Rica License.
Basada en una obra en http://www.revistas.una.ac.cr/index.php/revmar.
Permisos que vayan más allá de lo cubierto por esta licencia pueden encontrarse en revmar@una.cr.
BIBLIOGRAPHY
Aguirre, A. A., Balazs, G. H., Spraker, T. R. & Gross, T. S. (1995). Adrenal and haematological responses to stress in juvenile green turtles (Chelonia mydas) with and without fibropapillomatosis. Physiol. Zool., 68, 831-854.
Aguirre, A. A., Spraker, T. R., Chaves, A., Du Toit, L., Eure, W. & Balzs, G. H. (1999). Pathology of fibropapillomatosis in olive ridley turtles Lepidochelys olivacea nesting in Costa Rica. J. Aquat. Anim. Healt., 11(3), 283-289.
Aguirre, A. A. & Balazs, G. H. (2000). Blood biochemistry values of green turtles, Chelonia mydas, with and without fibropapillomatosis. Comp. Haematol. Int., 10, 132-137.
Araya, A., Rojas, M. & Villalobos, J. (2002). La explotación de los huevos de la tortuga en la región de Guanacaste, Refugio Nacional de Vida Silvestre Ostional. No publishing. Universidad Nacional, Costa Rica
Balazs, G. H. (1986). Fibropapillomas in Hawaiian green turtles. Mar. Turtle Newsletter, 39, 1-3.
Balasz, G. H. & Pooley, S. G. (Eds.). (1991). Research plan for marine turtle fibropapilloma. Washington, EE.UU.: Dep. Commer., NOAA Tech. Memo. NMFS-SWFSC-156.
Ballestero, J., Arauz, R. M. & Rojas, R. (1998, March). Management, conservation, and sustained use of olive ridley sea turtle eggs (Lepidochelys olivacea) in the Ostional Wildlife Refuge, Costa Rica: an eleven year review. Paper presented at the Proceedings of the Eighteenth International Sea Turtle Symposium. Sinaloa, Mexico.
Beynon, P. H. & Cooper, J. E. (1999). Reptiles. In O. F. Jackson (Ed.), Manual de animales exóticos (pp. 258-260). Madrid, Spain: Harcourt Brace de España, S. A.
Brooks, D. E., Ginn, P. E., Miller, T. R., Bramson, L. & Jacobson, E. R. (1994). Ocular fibropapillomas of green turtles (Chelonia mydas). Vet. Pathol., 31, 335-339.
Chaves, A., Du Toit, L., Marín, G. & Eure, W. (1998, March). Fibropapilloma in the Ostional olive ridley (Lepidochelys olivacea) population. Paper presented at the Proceedings of the Eighteenth International Sea Turtle Symposium. Sinaloa, Mexico.
Coberley, S. S., Condit, R. C., Herbst, L. H. & Klein, P. A. (2002). Identification and expression of immunogenic proteins of a disease-associated marine turtle herpesvirus. J. Virol., 76, 10553-10558.
Davidson, M. G., Else, R. W. & Lumsden, J. H. (Eds.). (1998). Collection and handling of samples for diagnosis. In R. W. Else (Ed.), BSAVA Manual of small animal clinical pathology (pp. 11-16). London, England: Iowa State University Press.
dos Santos, R. G., Silva, M. A., Torezani, E., Baptistotte, C., da Nóbrega, F. J., Antunes, H. P., Work, T. M. & Balazs, G. H. (2010). Relationship between fibropapillomatosis and environmental quality: a case study with Chelonia mydas off Brazil. Dis. Aquatic Organ., 89, 87-95.
Eckert, K. L., Bjorndal, K. A., Abreu-Grobois, F. A. & Donnelly, M. (Eds.). (2000). Técnicas de investigación y manejo para la conservación de las tortugas marinas. Pennsylvania, EE.UU.: Consolidated Graphic Communications.
Eckert, K. & Begge, J. (2006). Marine turtle tagging: a manual of recommended practices. North Carolina, EE.UU.: WIDECAST Technical report N° 2.
Ene, A., Su, M., Lemaire, S., Rose, C., Schaff, S., Moretti, R., Lenz, J. & Herbst, L. (2005). Distribution of chelonid fibropapillomatosis-associated herpesvirus variants in Florida: molecular genetic evidence for infection of turtles following recruitment to neritic developmental habitats. J. Wildl. Dis., 41(3), 459-497.
Forslund, O., Antonsson, A., Nordin, P., Stenquist, B. & Hansson, B. G. (1999). A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol., 80, 2437-2443.
Gámez, V. S., García, M. L., Osorio, S. D., Vázquez, G. J. & Constantino, C. F. (2009). Patología de las tortugas marinas (Lepidochelys olivacea) que arribaron a las playas de Cuyutlán, Colima, México. Vet. México, 40(1), 69-78.
GraphPad Software. (2013). GraphPad Software. Version 3.01 and GraphPad Prism version 3.0. www.graphpad.com/company/ California, EE.UU.: GraphPad Software, Inc.
Greenblatt, R., Quackenbush, S., Casey, R., Rovnak, J., Balasz, G., Work, T., Casey, J. & Suthon, C. (2005). Genomic variation of the fibropapilloma-associated marine turtle herpesvirus across seven geographic areas and three host species. J. Virol., 79(2), 1125-1132.
Herbst, L. H. (1994). Fibropapillomatosis of marine turtles. Annu. Rev. Fish. Dis., 4, 389-425.
Herbst, L. H., Jacobson, E. R., Moretti, R., Brown, T., Sundberg, J. P. & Klein, P. A. (1995). Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Organ., 22, 1-12.
Herbst, L. H., Greiner, E. C., Ehrhart, L. M., Bagley, D. A. & Klein, P. A. (1998). Serological association between spirorchidiasis, herpesvirus infection, and fibropapillomatosis in green turtles from Florida. J. Wildl. Dis., 34(3), 496-507.
Herbst, L. H., Jacobson, E. R., Klein, P. A., Balazs, G. H., Moretti, R., Brown, T. & Sundberg, J. P. (1999). Comparative pathology and pathogenesis of spontaneous and experimentally induced fibropapillomas of green turtles (Chelonia mydas). Vet. Pathol., 36, 551-564.
Herbst, L. H., Chakrabarti, R., Klein, P. A. & Achary, M. (2001). Differential gene expression associated with tumorogenicity of cultured green turtle fibropapilloma-derived fibroblasts. Can. Genet. Cytogen., 129(1), 35-39.
Jacobson, E. R., Mansell, J. L., Sundberg, J. P., Hajjar, L., Reichmann, M. E., Ehrhart, L. M., Walsh, M. & Murrus, F. (1989). Cutaneous fibropapillomas of green turtles (Chelonia mydas). J. Comp. Path., 101, 39-52.
Lackovich, J. K., Brown, D. R., Homer, B. L., Garber, R. L., Mader, D. R., Moretti, R. H., Patterson, A. D., Herbst, L. H., Oros, J., Jacobson, E. R., Curry, S. S. & Klein, P. A. (1999). Association of herpesvirus with fibropapillomatosis of the green turtle Chelonia mydas and the loggerhead turtle Caretta caretta in Florida. Dis. Aquat. Organ., 37, 89-97.
Lu, Y., Wang, Y., Yu, Q., Aguirre, A. A., Balazs, G. H., Nerurkar, V. R. & Yanagihara, R. (2000). Detection of herpesviral sequences in tissues of green turtles with fibropapilloma by polymerase chain reaction. Arch. Virol., 145, 1885-1893.
Lucke, B. (1938). Studies on tumors of cold-blooded vertebrates. Annu. Rep. Tortugas Lab. Carnegie Institute, Washington, D. C., 39, 92-94.
Meneses, A. I., Villalobos, J. E. & Sancho, E. (1993). Manual de hematología y química clínica en medicina veterinaria. Heredia, Costa Rica: EUNA.
Murray, M. J. (2000). Reptilian blood sampling and artifact considerations. In A. M. Fudge (Ed.), Laboratory Medicine Avian and Exotic Pets (pp. 187-188). Philadelphia, EE.UU.: W. B. Saunders.
Orós, J., Lackovich, J. K., Jacobson, E. R., Brown, D. R., Torrent, A., Tucker, S. & Klein, P. A. (1999). Fibropapilomas cutáneos y fibromas viscerales en una tortuga verde (Chelonia mydas). Rev. Esp. Herp., 13, 17-26.
Orrego, C. M. & Morales, J. A. (2002, April). Discoveries of olive ridley turtles (Lepidochelys olivacea) on the Pacific coast of Costa Rica. Paper presented at the 22nd Annual Symposium on Sea Turtle Biology and Conservation. Miami, Florida, EE.UU.
Orrego, C. M. & Work, T. (2004, February). Histopathological findings in olive ridley sea turtles (Lepidochelys olivacea) in Ostional and Nancite beaches in the Pacific Coast of Costa Rica. Paper presented at the 24th Annual Symposium on Sea Turtle Biology and Conservation. San José, Costa Rica.
Owens, D. W. (1980). The comparative reproductive physiology of sea turtles. Am. Zool., 20, 547-564.
Quackenbush, S. L., Work, T. M., Balazs, G. H., Casey, R. N., Rovnak, J., Chaves, A., Du Toit, L., Baines, J. D., Parrish, C. R., Bowser, P. R. & Casey, J. W. (1998). Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virol., 246, 392-399.
Quackenbush, S. L., Casey, R. N., Murcek, R. J., Paul, T. A., Work, M., Limpus, C. J., Chaves, A., Du Toit, L., Vasconcelos, P. J., Aguirre, A. A., Spraker, T. R., Horrocks, J. A., Vermeer, L. A., Balazs, G. H. & Casey, J. W. (2001). Quantitative analysis of herpesvirus sequences from normal tissue and fibropapillomas of marine turtles with real-time PCR. Virol., 287, 105-111.
Rodenbusch, C. R., Almeida, L. L., Marks, F. S., Ataíde, M. W., Alievi, M. M., Tavares, M., Pereira, R. A. & Canal, C. W. (2012). Detection and characterization of fibropapilloma associated herpesvirus of marine turtles in Rio Grande do Sul, Brazil. Pesquisa Vet. Brasil, 32(11), 1179-1183.
Santoro, M. & Meneses, A. (2007). Haematology and plasma chemistry of breeding olive ridley sea turtles (Lepidochelys olivacea). Vet. Rec., 161, 818-819.
Santoro, M., Morales, J. A. & Rodríguez-Ortiz, B. (2007). Spirorchiidiosis (Digenea: Spirorchiidae) and lesions associated with parasites in Caribbean green turtles (Chelonia mydas). Vet. Rec., 161, 482-486.
Smith, G. M. & Coates, C. (1938). Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas. Zool., 23, 93-98.
Vale, O. E. & Bracho, A. (2000). Fibropapilomas en una tortuga marina (Chelonia mydas): morfología macro y microscópica en un caso capturado en la península de Paraguaná, Estado Falcón, Venezuela. Rev. Cient. FCV-Luz., 5, 367-371.
Valverde, R., Orrego, C. M., Tordoir, M. T., Gómez, F. M., Solís, D. S., Hernández, R. A., Gómez, G. B., Brenes, L. S., Baltodano, J. P., Fonseca, L. G. & Spotila, J. R. (2012). Olive ridley mass nesting ecology and egg harvest at Ostional Beach, Costa Rica. Chelonian Conserv. Biol., 11(1), 1-11.
Work, T. & Balazs, G. (1999). Relating tumor score to hematology in green turtles with fibropapillomatosis in Hawaii. J. Wildlife Dis., 35(4), 804-807.