Rev. Mar. Cost. ISSN 1659-455X. Vol. 6: 103-113, Diciembre 2014.
DOI: http://dx.doi.org/10.15359/revmar.6.7
Usefulness of meiofauna at high taxonomic levels as a tool to assess harbor quality status. Marina del Este Harbor (Granada, Spain) as a case study
Utilidad de la meiofauna a niveles taxonómicos altos como herramienta para evaluar el estado de calidad en puertos. El puerto de Marina del Este (Granada, España) como caso de estudio
Francisco Sedano1 *, Daniel Marquina2 & Free Espinosa1
1 Laboratorio de Biología Marina. Universidad de Sevilla. Avda. Reina Mercedes 6. 41012. Sevilla, Spain. Tno: +34-954556229.
2 Departamento de Zoología y Antropología Física. Universidad Complutense de Madrid. Ciudad Universitaria. 28040. Madrid, Spain. free@us.es f.sedano.vera@hotmail.com*
Recibido: 24 de marzo de 2014
Corregido: 7 de junio de 2014
Aceptado: 16 de julio de 2014
ABSTRACT
Meiofaunal communities from a harbor with low levels of disturbance (Marina del Este Harbor) and a control area (Galera Beach) at the subtropical coast of Almuñecar (Spain) were compared to evaluate the usefulness of meiofauna as a tool for environmental monitoring at high taxonomic levels in harbors. Three sites were sampled at the control area with different granulometries (Fine, Medium and Coarse). A mixture of fine and medium sands were found in the Medium granulometry site, which had the same granulometric composition as the Harbor Site. Al, Ba, Ca, Mg, Mn and Ni concentrations at the harbor site were twice as high as those found in the Medium Control Site, which correlates with the highest organic content. Results of the one-way ANOVA and the multivariate analysis showed significant differences regarding the meiofauna abundance between the Medium and Harbor Sites. In conclusion, the study of meiofaunal communities at high taxonomic levels can detect low levels of disturbance in harbors, which makes it a useful tool for preliminary assessment of environmental quality and, therefore, can be implemented in management policies.
Keywords: Harbor, meiofauna, Alboran Sea, environmental quality, environmental monitoring.
RESUMEN
Las comunidades de meiofauna de un puerto poco perturbado (puerto de Marina del Este) y una zona control (Playa Galera) en la costa subtropical de Almuñécar (España) fueron comparadas para evaluar la utilidad de la meiofauna a niveles taxonómicos superiores como herramienta de monitoreo ambiental en puertos. En la zona control se muestrearon tres estaciones con diferentes granulometrías (Fina, Media y Gruesa). Se encontró una mezcla de arenas medias y finas en la estación de granulometría Media, la cual tuvo la misma composición granulométrica que la estación Puerto. Las concentraciones de Al, Ba, Ca, Mg, Mn y Ni de la estación Puerto fueron dos veces superiores a las encontradas en la estación control Medio, correlacionándose con el mayor contenido orgánico. Los resultados del ANOVA de una vía, así como los análisis multivariados, mostraron diferencias significativas en la abundancia de meiofauna entre las estaciones Medio y Puerto. Se concluye que el estudio de la meiofauna a niveles taxonómicos superiores puede detectar niveles bajos de perturbación en puertos, siendo una herramienta útil para la evaluación preliminar de la calidad ambiental en puertos, por lo que puede ser implementada en las políticas de gestión.
Palabras claves: Puerto, meiofauna, Mar de Alborán, estado de calidad, monitoreo ambiental.
INTRODUCTION
Over the last thirty years numerous studies on meiofauna have increasingly proven the role of these small animals in marine sediments, indirectly because of bioturbation processes (exposing buried sediments) and the induction of bacterial metabolism, and directly because animals in higher trophic levels feed on them, largely determining their abundance and distribution. Their small size, high abundance, rapid generation times and absence of a planktonic phase give them an important role in the decomposition of detritus in the cycle of nutrients and energy flow (Green & Montagna, 1996; Higgins & Thiel, 1988; Warwick, 1987). Therefore, meiofauna can be a sensitive tool for the assessment of ecological conditions (Giere, 2009; Goodsell et al. 2009; Moreno et al. 2011).
According to the Water Framework Directive of the European Union (WFD, Directive 200/60/EC), biological descriptors are essential for evaluating and monitoring environmental conditions in order to ensure effective protection strategies and management of aquatic systems. Popularly, macrofauna assemblages have been used as sensitive tools to assess local pressure. However, the shorter generation times of the meiofauna and the absence of a planktonic phase suggest a potentially shorter response in time and, therefore, a higher sensitivity to anthropogenic stress (Coull & Chandler, 1992; Heip et al. 1988; Warwick, 1993) than macrofauna. However, meiofauna indexes (such as the nematode/copepod ratio) are not yet within the scope of the WFD.
In harbor systems, where macrofauna is scarce and difficult to sample effectively, the study of meiofaunal assemblages arises as the best instrument for monitoring purposes. Ports, ranging from large commercial harbors to small tourist marinas, represent the main link between anthropized and natural coastal ecosystems, and, consequently, should be taken into account as one of the main sources of coastal disturbances. Harbor management policies have been developed over the last years and their implementation is promoted through awards such as the Blue Flag Award (The Foundation for Environmental Education, 1987).
In this sense, surveys should be conducted periodically, thus giving easy protocols a significant advantage. Previous studies already tried to simplify protocols. For instance, Moreno et al. (2008) analyzed both meiofauna higher taxa and nematode communities at the genus level. They concluded that, in three Mediterranean harbors, nematode genus-based indicators correlated with contaminant concentrations at levels of significance similar to those used for meiofauna higher taxa, therefore suggesting that a more detailed taxonomic resolution did not necessarily improve the final outcome of environmental quality assessments in these harbors. Similar studies support this hypothesis (see Warwick, 1988; Kennedy & Jacoby, 1999; Mirto et al. 2010). Consequently, a simpler method is suggested here based on the study of community structures at high taxonomic levels. Since the aim of this study is to reinforce the use of meiofauna as a tool for environmental monitoring and more specifically to present the basis for an easy and fast preliminary approach to assess harbor ecosystem quality status, a small tourist marina and a control site were selected at the subtropical coast of Granada (Alboran Sea). By selecting a small marina, the aim is to test how useful this method is to detect induced stress at very low rates of pollution. Positive results will also help the implementation of meiofauna procedures between the scope of the WFD or other local management policies in greater harbors worldwide.
MATERIAL AND METHODS
Study site and sampling methods
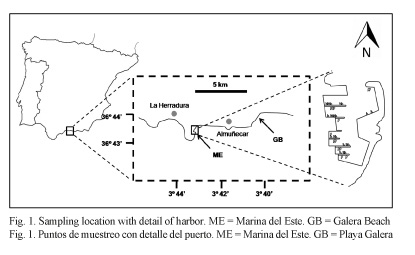
Two points in the vicinity of Almuñecar (Granada, Spain) were used in the study: a control area established at Galera Beach and a harbor with low levels of disturbance, Marina del Este (Fig. 1). The currents on the north side of the permanent geostrophic gyre of the Alboran Sea generate nutrient upwelling along this coast (Templado et al., 2006), making it a very interesting study area. Marina del Este harbor is a small tourist marina with 3.7 m average depth, 277 moorages and a uniform granulometric composition of medium size sands. The control area is a semi-isolated sandy beach with 3 m average depth, well defined granulometric gradient (from fine to coarse including the same grain size as the harbor station), and the lowest rate of anthropogenic stress from the surrounding beaches, which made it the most suitable control area for this study. At the control area three sites were randomly sampled based on their granulometric class: “Fine”, “Medium” and “Coarse”, separated each other by tens of meters. At Marina del Este harbor (referred to as “the Harbor”), only three sites were sampled as there was only one granulometric class. In November 2012, three replicates were taken at each site via scuba diving using cylindrical cores of 4 cm diameter and 10 cm depth in the sediment. In order to analyze granulometry and chemical parameters, two additional samples were collected for each granulometric class.
Physicochemical analysis
Granulometric parameters were determined following the method proposed by Guitián & Carballas (1976). Sediment samples destined for chemical analyses were immediately frozen at -20ºC pending further laboratory analyses. Under laboratory conditions, sediment samples were air-dried, crushed and sieved through a 2 mm sieve and then ground to <60 µm. The sediments were chemically analyzed for organic matter (OM), total organic carbon (TOC), total nitrogen (N) and metal trace elements. OM and TOC were analyzed by dichromate oxidation and titration with ferrous ammonium sulphate (Walkley & Black, 1934). N was determined by the Kjeldahl digestion method (Hesse, 1971). For the analysis of metal and trace element concentrations in sediments, samples were digested with aqua regia (1:3 concHNO3:HCl) in a microwave digester. Quantification of elements in the extracts was achieved using a VARIAN ICP 720-ES (simultaneous ICP-OES with axially viewed plasma). The accuracy of the analytical methods was assessed through the BCR analysis (Community Bureau of Reference) and a reference soil sample from the Wageningen Evaluating Programs for Analytical Laboratories for soils, International Soil-analytical Exchange (WEPAL, ISE).
Meiofaunal analysis
Each sample was mixed with magnesium chloride 7.5% MgCl2 in distilled water and, after 10 minutes, sieved through a 0.5 mm sieve (in order to retain macrobenthos) and a 30 µm sieve (in order to obtain meiofauna). This process was repeated three times for each sample and, subsequently, the animals were fixed in 70% ethanol and stained with Rose Bengal. In the laboratory, the meiofaunal specimens were identified at a high taxonomic level (phylum, class or order) under a stereomicroscope.
Statistical analysis
The mean and standard error of abundance of each taxa were calculated for each sampling site. In order to test the null hypothesis of no change in meiofauna abundances between sites, two different approaches (univariate and multivariate analyses) were applied.
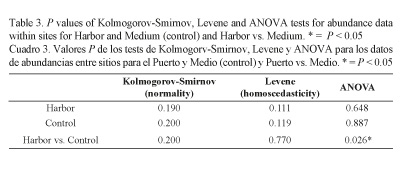
To test whether the abundances of replicates were similar between and within the Harbor and control areas (Medium granulometry), a one-way analysis of variance (ANOVA) was conducted using SPSS (2006), after verifying that data was normally distributed (Kolmogorov-Smirnov test) and complied with assumption of homogeneity of variances (Levene’s test).
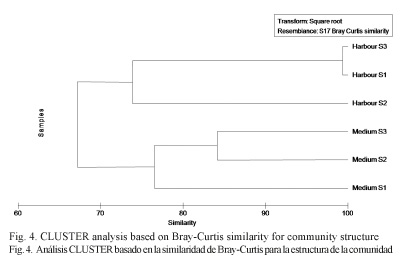
Multivariate MDS (non-metric multidimensional scaling) statistics were also used based on the UPGMA method (Unweighted Pair-Group Method using arithmetic averages), along with the Bray–Curtis similarity index. MDS was used to test differences in community structures among control and harbor areas. Kruskal’s stress coefficient was used to test the ordination (Kruskal & Wish, 1978). The data was fourth root transformed. In the same way, Cluster analysis based on the Bray-Curtis was also performed. Multivariate analyses were carried out using the PRIMER v.6+PERMANOVA package (Clarke, 1993).
RESULTS
At control sites, a total of 15 higher taxa were identified, 7 of which were always present. By contrast, at harbor sites, a total of 10 higher taxa were identified, lacking the presence of Nemertina, Kynorhyncha, Tardigrada and Acarina that were found at the control site. Tanaidacea was the only harbor-specific group (Table 1).
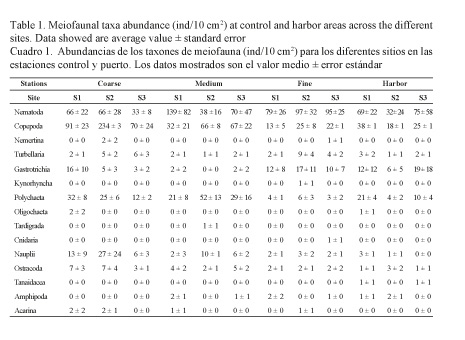
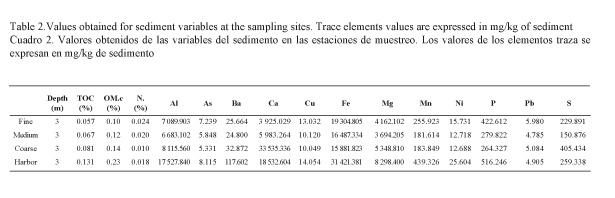
Granulometric data for the control area showed a prevalence of fine sands (0.063-0.25 mm) for Fine sites, a prevalence of gross sands (0.5-2 mm) for Coarse sites and a mixture of fine and medium sands (0.25-0.5 mm) for Medium sites, which in turn, had the same granulometric composition as the Harbor area (Fig. 2). Results concerning other sediment variables such as TOC, OM, N and trace elements are reported in Table 2. Levels of TOC, OM and N were low and similar between control sites. Levels of these variables at the Harbor area were also low but slightly higher for TOC and OM than control ones, correlating with the trace element values that were two fold higher for Al, Ba, Ca, Mg, Mn and Ni at the Harbor area compared to Medium sites at the control area (Table 2).
Since the Harbor area and the Medium sites at the control area showed similar depths and granulometric compositions, they were selected to assess possible disturbances on meiofauna abundances. Results of the one-way ANOVA comparing abundances are shown in Table 3. No significant differences were recorded within the Harbor and Medium sites. However, the latter showed significantly higher abundance than the Harbor.
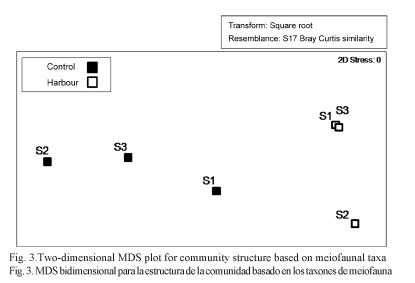
A two dimensional MDS plot portrayed differences in community structure between the Harbor and Medium sites showing a divergence between them (Fig. 3). In a similar way, the CLUSTER analysis showed the same differences gathering the Harbor and Medium sites in two differentiated groups with over 70% similarity (Fig. 4).
DISCUSSION
Methods for assessing environmental quality status in Mediterranean coastal ecosystems and in harbors through the analysis of meiofauna composition have been already suggested by Moreno et al. (2008; 2011). Unlike those research works, which use genus-based indicators and, therefore, require training and expertise in the matter, our study demonstrates that environmental degradation can also be assessed through the analysis of differences at a coarse taxonomic level. Meiofaunal genus-based indices usually use nematodes as a model organism, not only for being the most abundant taxa in the meiobenthos, but also for their important role within trophic webs (Goodsell et al., 2009; Kennedy & Jacoby, 1999; Platt & Warwick, 1980). The use of genus-based indices, especially those of nematodes, is a time demanding and very specialized task that only experts can carry out. Without a doubt, such detailed study of the sediment provides very valuable information. However, this paper suggests a faster and easier method for assessing quality status, which could therefore serve as a preliminary study for determining if further and more detailed analyses are required. Thus, meiofauna should be used as a tool for environmental assessment and as a basis for a simple and fast approach to evaluate harbor ecosystem quality status.
Sediment structure is a major factor influencing community structure (Conrad, 1976; Heip et al., 1985; Wieser, 1959), thus the selection of stations with the same physical composition allowed to leave such differences at a chemical level, making data interpretation easier. Moreover, trace elements data at the control station are concordant with data from Navarro-Barranco et al. (2012), collected at the Maro Cerro Gordo Nature Reserve, an undisturbed area close to our control site. These similar values suggest a conserved pattern for chemical variables within undisturbed areas at the subtropical coast of Alboran Sea. At the Harbor area, Al, Ba, Ca, Mg, Mn and Ni were two-fold higher, which could explain the lack of some groups in this area. Even though the disturbance level in the harbor was not very high (see ranges of chemical parameters in harbors by Guerra-García & García-Gómez, 2005), significant differences were detected in the meiofauna community. Although other harbors should be analyzed, this method could be very suitable for detecting changes on a small scale at tourist marinas. Moreover, in extensive commercial harbors with higher concentrations of pollutants due to intense shipping activities (see Guerra-García & García-Gómez, 2005), this method is expected to be even more accurate.
In summary, the relatively easy identification of meiofauna at a high taxonomic level provides the opportunity to make a quick survey that can be conducted by non-experts in the field. Therefore, its implementation in the WFD or local management policies would be of great interest.

Usefulness of meiofauna at high taxonomic levels as a tool to assess harbor quality status. Marina del Este Harbor (Granada, Spain) as a case study por Revista Ciencias Marinas y Costeras se distribuye bajo una Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Costa Rica License.
Basada en una obra en http://www.revistas.una.ac.cr/index.php/revmar.
Permisos que vayan más allá de lo cubierto por esta licencia pueden encontrarse en revmar@una.cr.
BIBLIOGRAPHY
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Eco., 18, 117-143. DOI: http://dx.doi.org/10.1111/j.1442-9993.1993.tb00438.x
Conrad, J. E. (1976). Sand grain angularity as a factor affecting colonization by marine meiofauna. Vie Milieu B., 26, 81-198.
Coull, B. C. & Chandler, G. T. (1992). Pollution and meiofauna: field, laboratory, and mesocosms studies. Oceanogr. Mar. Biol. Ann. Rev., 30, 191-271.
Giere, O. (2009). Meiobenthology. The microscopic motile fauna of aquatic sediments. (2nd ed.). Berlin, Germany: Springer.
Goodsell, P. J., Underwood, A. J. & Chapman, M. G. (2009). Evidence necessary for taxa to be reliable indicators for environmental conditions or impacts. Mar. Pollut. Bull., 58(3), 323-331. DOI: http://dx.doi.org/10.1016/j.marpolbul.2008.10.011
Green, R. H. & Montagna, P. (1996). Implications for monitoring: study designs and interpretation of results. Can. J. Fish. Aquat. Sci., 53(11), 2629-2636. DOI: http://dx.doi.org/10.1139/f96-219
Guerra-García, J. M. & García-Gómez, J. C. (2005). Assessing pollution levels in sediments of a harbor with two opposing entrances. Environmental implications. J. Environ. Manage., 77, 1-11. DOI: http://dx.doi.org/10.1016/j.jenvman.2005.01.023
Guitián, F. & Carballas, T. (1976). Técnicas de análisis de suelos (2nd ed.). Santiago de Compostela, Spain: Pico Sacro.
Heip, C., Warwick, R. M., Carr, M. R., Herman, P. M. J., Huys, R., Smol, N. & Holsbeke, K. V. (1988). Analysis of community attributes of the benthic meiofauna of Frierfjord/Langesundfjord. Mar. Ecol. Prog. Ser., (46), 171-180. DOI: http://dx.doi.org/10.3354/meps046171
Heip, C., Vincx, M. & Vranken, G. (1985). The ecology of marine nematodes. Oceanogr. Mar. Biol. Ann. Rev., 23, 399-489.
Hesse, P. R. (1971). A Textbook of soil chemical analysis. London, United Kingdom: John Murray.
Higgins, R. P. & Thiel, H. (1988). Introduction to the study of meiofauna. Washington, D. C. USA.: Smithsonian Institution Press.
Kennedy, A. D. & Jacoby, C. A. (1999). Biological indicators of marine environmental health: meiofauna - a neglected benthic component? Environ. Monit. Assess., 54, 47-68. DOI: http://dx.doi.org/10.1023/A:1005854731889
Kruskal, J. B. & Wish, M. (1978). Multidimensional Scaling. Sage University paper series on quantitative applications in the social sciences. Beverly Hills and London, USA. & U.K.: Sage Publications.
Mirto, S., Bianchelli, S., Gambi, C., Krzelj, M., Pusceddu, A., Scopa, M., Holmer, M. & Danovaro, R. (2010). Fish-farm impact on metazoan meiofauna in the Mediterranean Sea: Analysis of regional vs. habitat effects. Mar. Environ. Res., 69(1), 38-47. DOI: http://dx.doi.org/10.1016/j.marenvres.2009.07.005
Moreno, M., Semprucci, F., Vezzulli, L., Balsamo, M., Fabiano, M. & Albertelli, G. (2011). The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol. Indic., 11(2), 328-336. DOI: http://dx.doi.org/10.1016/j.ecolind.2010.05.011
Moreno, M., Vezzulli, L., Marin, V., Laconi, P., Albertelli, G. & Fabiano, M. (2008). The use of meiofauna diversity as an indicator of pollution in harbors. ICES J. Mar. Sci., 65(8), 1428-1435. DOI: http://dx.doi.org/10.1093/icesjms/fsn116
Navarro-Barranco, C., Guerra-García, J. M., Sánchez-Tocino, L. & García-Gómez, J. C. (2012). Soft-bottom crustacean assemblages in Mediterranean marine caves: the cave of Cerro Gordo (Granada, Spain) as case study. Helgol. Mar. Res., 6(4), 567-576. DOI: http://dx.doi.org/10.1007/s10152-012-0292-5
Platt, H. M. & Warwick, R. M. (1980). The significance of free-living nematodes to the littoral ecosystem. In J. H. Price, D. E. G. Irvine & W. F. Farnham (Eds.), The shore environment: Ecosystems (pp. 729-759). New York, USA.: Academic Press.
SPSS. (2006). Statistical Package for the Social Sciences for Windows, version 15.0. Chicago, USA.: SPSS Inc.
Templado, J., Calvo, M., Moreno, D., Flores-Moya, A., Conde, F., Abad, R., Rubio, J., López-Fé, C. M. & Ortiz, M. (2006). Flora y fauna de la reserva marina y reserva de pesca de la isla de Alborán. Madrid, Spain: Ministerio de Agricultura, Pesca y Alimentación. Secretaría General de Pesca Marítima.
The Foundation for Environmental Education. (1987). Blue Flag Programme. Retrieved on May 4, 2013 available at http://www.fee-international.org/en/menu/programmes/blue-flag
Walkley, A. & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci., 37, 29-38. DOI: http://dx.doi.org/10.1097/00010694-193401000-00003
Warwick, R. M. (1987). Meiofauna: their role in marine detrital systems. In D. J. W Moriarty & R. S. V. Pullin (Eds.), Detritus and microbial ecology in aquaculture (pp. 282-295). Manila, Philippines: ICLARM Contribution.
Warwick, R. M. (1988). The level of taxonomic discrimination required to detect pollution effects on marine benthic communities. Mar. Pollut. Bull., 19(6), 259-268. DOI: http://dx.doi.org/10.1016/0025-326X(88)90596-6
Warwick, R. M. (1993). Environmental impact studies on marine communities: pragmatic considerations. Aust. J. Ecol., 18, 63-80. DOI: http://dx.doi.org/10.1111/j.1442-9993.1993.tb00435.x
Wieser, W. (1959). The effect of grain size on the distribution of small invertebrates inhabiting the beaches of Puget Sound. Limnol.Oceanogr., 4(2), 181-194. DOI: http://dx.doi.org/10.4319/lo.1959.4.2.0181