Rev. Mar. Cost. ISSN 1659-455X. Vol. 7: 83-98, Diciembre 2015.
DOI: http://dx.doi.org/10.15359/revmar.7.6
Reproduction of Gerres cinereus (Percoidei: Gerreidae) off the Mexican Pacific coast
Reproducción de Gerres cinereus (Percoidei: Gerreidae) en la costa del Pacífico mexicano
Elaine Espino-Barr1*, Manuel Gallardo-Cabello2, Esther G. Cabral-Solís1, Marcos Puente-Gómez1 & Arturo García-Boa1
1 Instituto Nacional de Pesca, Playa Ventanas s/n, Manzanillo, Colima, México; elespino@gmail.com*
2 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, México, D. F.;
gallardo@cmarl.unam.mx
Recibido: 4 de diciembre de 2014
Corregido: 21 de abril de 2015
Aceptado: 20 de julio de 2015
ABSTRACT
Yellowfin Mojarra Gerres cinereus (Walbaum, 1792) off the Mexican Pacific coast is a popular low-cost species, which is caught with gill and cast nets. Knowing breeding seasons, gonadal maturity, fecundity and size at sexual maturity is necessary for fishery management. Samples were obtained from April 2010 to November 2011. Size, sex and gonad maturity were recorded, while the gonadosomatic index and the fecundity and condition factors were calculated using the gravimetric method. To calculate this information by age, growth parameters previously published were used. The female:male ratio was 1:1.024. Mature females were observed year round with two major peaks during July and October. Sexual maturation (L0.5) of males and females was 16.40 cm and 20.20 cm, respectively, corresponding to one-year old organisms. First maturity length (L0.25) was 15.80 cm in males and 16.50 cm in females. The hepatosomatic index vs length was LW = 2.00·10-6 ·TL4.213, resulting in a positive allometric relationship. Condition factor indexes increased during February and September. Average oocyte diameter was 0.31 mm (0.15 to 0.45 mm). Fecundity ranged from 37,784 to 1,746,510 oocytes in females from one to seven years of age, and mean relative fecundity was 1332 oocytes·g-1 (294 to 4,430 oocytes·g-1). G. cinereus has an early sexual maturity, reproduces once or twice a year and has a high fecundity rate, which allows it to be caught all year round, if caught after the first maturity length.
Keywords: Fecundity, maturity period, fish reproduction, minimum spawning size, yellowfin mojarra.
RESUMEN
La mojarra rayada Gerres cinereus (Walbaum, 1792) del Pacífico mexicano se captura con redes agalleras y atarrayas, es una especie popular de bajo costo. Para el manejo de la pesquería es necesario conocer las épocas de reproducción, madurez gonádica, fecundidad y talla de primera madurez sexual. Las muestras se obtuvieron de abril de 2010 a noviembre de 2011. Se registraron longitudes, sexo y madurez gonadal, se calcularon índice gonadosomático, factor de condición y fecundidad por el método gravimétrico. Para calcular esta información por edad se utilizaron los parámetros de crecimiento publicados anteriormente. La relación hembra:macho fue 1: 1.024. Se observaron hembras maduras todo el año, con máximos desoves en julio y octubre. La longitud promedio de reproducción (L0.5) de machos fue 16.40 cm y de hembras 20.20 cm, que corresponde a organismos de un año de edad. La longitud de primera madurez (L0.25) fue 15.80 cm en machos y 16.50 cm en hembras. El índice hepatosomático vs. longitud fue LW = 2.00·10-6 ·Lt4.213, resultando alométrica positiva. Los factores de condición mostraron incrementos en febrero y septiembre. El diámetro promedio del ovocito fue 0.31 mm (0.15 - 0.45 mm). El intervalo de la fecundidad fue 37 784 a 1 746 510 ovocitos en hembras de uno a siete años de edad y la media relativa 1 332 ovocitos·g-1 (294 a 4 430 ovocitos·g-1). G. cinereus madura sexualmente temprano, se reproduce una o dos veces al año y tiene una tasa de fecundidad elevada, que permite que se pesque todo el año, si se captura después de la primera reproducción.
Palabras claves: Fecundidad, periodo de madurez, reproducción del pez, talla mínima de reproducción, mojarra rayada.
INTRODUCTION
The Yellowfin Mojarra Gerres cinereus (Walbaum, 1792) occurs in the Western Atlantic and Eastern Pacific Oceans from Baja California to Peru. Its habitat is sandy bottoms close to reefs but it also inhabits brackish coastal lagoons. Juveniles form big schools. This species is omnivorous and feeds on vegetable matter, small benthic invertebrates and insects (Allen & Robertson, 1994; Bussing, 1995).
In Mexican waters, G. cinereus is caught with gill and cast nets. It is registered in the official statistics together with other species of the Gerreidae family. In 2011, landings of Gerreidae mojarras in Mexico decreased from 81,250 t in 2010, of which 63% was caught off the Pacific coast, to 62,668 t. A total of 8022 t was captured off Colima and Jalisco (SAGARPA, 2012). Within artisanal fishery, G. cinereus represents 1.5% of total weight of 146 species of fish landed for commercial purposes. Its price at the beach (when brought to shore) is $50.00 Mexican pesos per kilogram ($3.50 US dollar).
The reproduction of the Gerreidae family has been researched by García-Cagide et al. (1994) and García-Cagide et al. (1999) in Cuba; Sarre et al. (2005) in southern Australia; Iqbal et al. (2007) and Kanak & Tachihara (2008) in Japan; Ferrer-Montaño (2009) in South America, particularly Venezuela; López-Martínez et al. (2011) in Northern Mexican Pacific and Valdez-Zenil et al. (2013) in southern Mexico.
The importance of reproduction studies lies in the knowledge acquired regarding population dynamics and feedback mechanism of a species, which allows for the continuity of a species in time and space through the recruitment of new organisms. If the reproduction mechanism is interrupted by capturing individuals that have not yet reproduced at least once in their lifetime, the population may end up in danger of extinction. With the information obtained from captured organisms that have reproduced, maximum sustainable yield models and capture predictions can be used. In addition, reproduction studies allow establishing closed periods (Gallardo-Cabello et al. 2007; Espino-Barr et al. 2012).
Therefore, the objectives of the present study are to analyze in detail the reproductive cycle of G. cinereus and to learn massive spawning times as well as fecundity values, which will give a solid background for closed seasons and gill net mesh sizes, based on the minimum reproductive size. These fishing measures will allow the species to reproduce at least once, protecting fishery from overexploitation.
MATERIALS AND METHODS
Study site: From April 2010 to November 2011, G. cinereus samples were collected monthly from the commercial capture of coastal fishery in Manzanillo, Colima, Mexico (19° 00’ - 19° 02’ North Latitude and 104° 10’ - 104° 21’ West Longitude) and Tomatlán, Jalisco, Mexico (19° 58’ - 20° 04’ N and 105° 26’ - 105° 32’ W) (Fig. 1). Fish were collected at sunrise and sunset. Total length (TL, cm) and total weight (TW, g) were recorded for 427 individuals (fishermen deliver this species intact). Of those individuals, 179 were transported to the laboratory of the National Fisheries Institute in Mexico, where total length (TL, cm), total weight (TW, g), eviscerated weight (EW, g), and sex were recorded macroscopically for each specimen.
Sexual and gonad maturation were determined in visu on fresh organisms taken to the laboratory the same day they were caught. The stages of sexual maturity were determined using the phases described in Sokolov and Wong (1973), Holden and Raitt (1975), Aboussouan and Lahaye (1979) and Espino-Barr et al. (2008): Phase I: undefined, sexual glands are a fine filament, and females and males cannot be differentiated. Phase II: Immature, the gonads start developing, ovaries are rose translucent and testes resemble a whitish lace. Oocytes can be observed. Phase III: Maturing, sexual glands are well differentiated. Ovaries look granular and are pink-yellowish; oocytes are small and opaque and still forming; testes are ivory white. Phase IV: Mature, sexual glands are well developed, ovaries are rose-orange, oocytes are big and transparent, and testes are whitish. Phase V: Spawning, ovaries are brilliant orange; sexual products are ready to be expelled and are pushed out at the slightest pressure, veins are well developed irrigating the entire gonad; testes are pearly white, sperm emerges at a light pressure. Phase VI: Post-spawn, product has been expelled; sexual glands are flaccid, swelled and brownish-gray. Residual oocytes are reabsorbed.
The first spawning TL for males and females was determined by 50% of the accumulative frequency (L50) of stages IV and V of sexual maturation (Sparre & Venema, 1995), and the minimum TL of first spawning (L25) was also recorded to be compared with other authors’ findings (Rodríguez-Gutiérrez, 1992). These two stages were considered because they are the closest stages to reproduction. The logistic function was described by the following equation (Gaertner & Laloe, 1986; Sparre & Venema, 1995):
, ![]()
where: HP = percentage of females or males sexually mature, a and b are constants. Its logarithmic transformation is:
![]()
and the length at which 50% of the population is sexually mature (L0.5) corresponds to:
![]()
The original equation is modified to include L0.5:
![]()
The minimum TL of first spawning (L25) was also recorded to be compared with other authors’ findings.
The gonadosomatic index (GSI) for females and males was calculated according to Rodríguez-Gutiérrez (1992), where gonad weight (GW) is expressed as a function of body weight: GSI = 100·GW/TW (TW = total weight). To measure physical fitness of fish, we obtained the condition factor K = (EW·TL-3)*100 (Clark, 1928), K = (TW·TL-3)*100 (Fulton, 1902) and a = TW·TL-b and a = EW·TL-b (Safran, 1992), the hepatosomatic index (HSI), expressed as the percentage of liver weight (LW) with respect to the total weight HSI = 100·LW/TW (Rodríguez-Gutiérrez, 1992). The stomach repletion index is the relation between the stomach weight and the body weight, calculated individually and averaged monthly.
Fecundity (F) and relative fecundity were obtained by the gravimetric method using the wet weight of 20 phase V female gonads of G. cinereus. To estimate total fecundity, two subsamples of 0.1 g were obtained of each individual and put in modified Gilson fluid (Simpson, 1951) for preservation. All oocytes were counted with the help of a stereoscopic microscope and measured with an ocular micrometer.
The following expression was used in the calculation: F = n · Gi/gi, where F = fecundity of a sample; n = number of oocytes in the subsample; Gi = weight of the gonad (g) and gi = weight of the subsample (g) (Holden & Raitt, 1975). The relationship between fecundity and total length and weight was calculated with the formula F = a·xb where F= fecundity, x = individual weight or length, a = intercept or initial number of oocytes, b = slope or oocyte number changing rate.
The relationships between TL, TW, LW, testis weight (TeW), ovary weight (GW), and fecundity were defined for different ages, using published growth parameters, obtained by sagittal otolith analysis (Espino-Barr et al. 2014; 2015).
RESULTS
Since G. cinereus cannot be differentiated by their body morphology, they have to be opened and eviscerated; therefore, only 179 organisms were sexed. Using gill nets of different size (2.5-3.0 in) in commercial fishery allowed catching individuals of various age groups.
Gonads were differentiated macroscopically, except for the immature individuals who had never spawned. Ovaries are cylindrical and, when matured, oocytes turn yellowish orange and are easily observed. Table 1 shows values of the gonad weight (GW, g), length (TL, cm), and total weight (TW, g), eviscerated weight (EW, g), liver weight (LW, g), and fecundity (number of oocytes), for each age group.
Testes are elongated, whitish and smaller than the ovaries. Table 1 shows that in, 4 year-old individuals, ovaries are 17.50 g, while testes are 12.90 g.
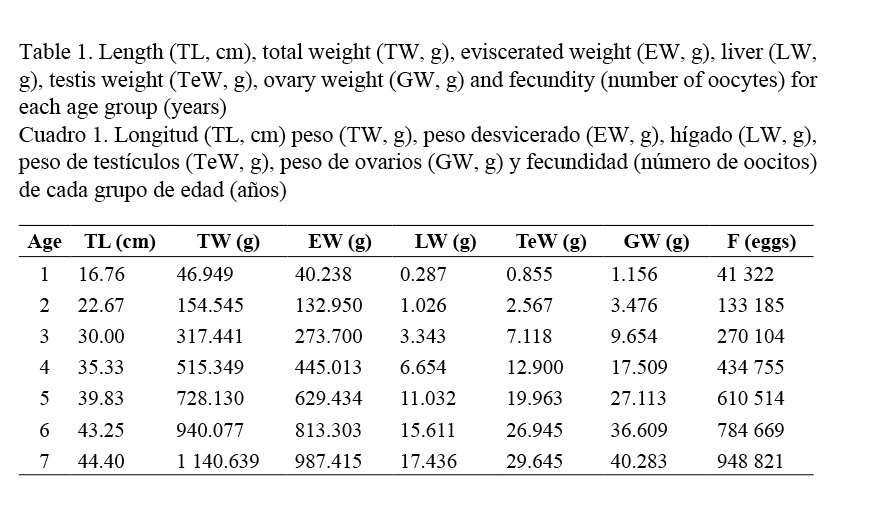
Oocyte diameters were 0.31 mm (±0.70 mm standard deviation: SD), minimum 0.15 mm and maximum 0.45 mm. Fecundity values ranged from 37,784 to 1,746,510 oocytes in females from one to seven years of age, length from 16.80 cm to 44.49 cm, and weight 46.95 g to 1140 g (Table 1). The average value of relative fecundity was of 1332 oocytes·g-1 (ranging from 294 to 4430 oocytes·g-1).
The sample included 178 organisms of Gerres cinereus, of which 85 were female (47.75%), 83 male (46.63%), and 10 undetermined (5.62%). The male: female proportion was 1: 1.024.
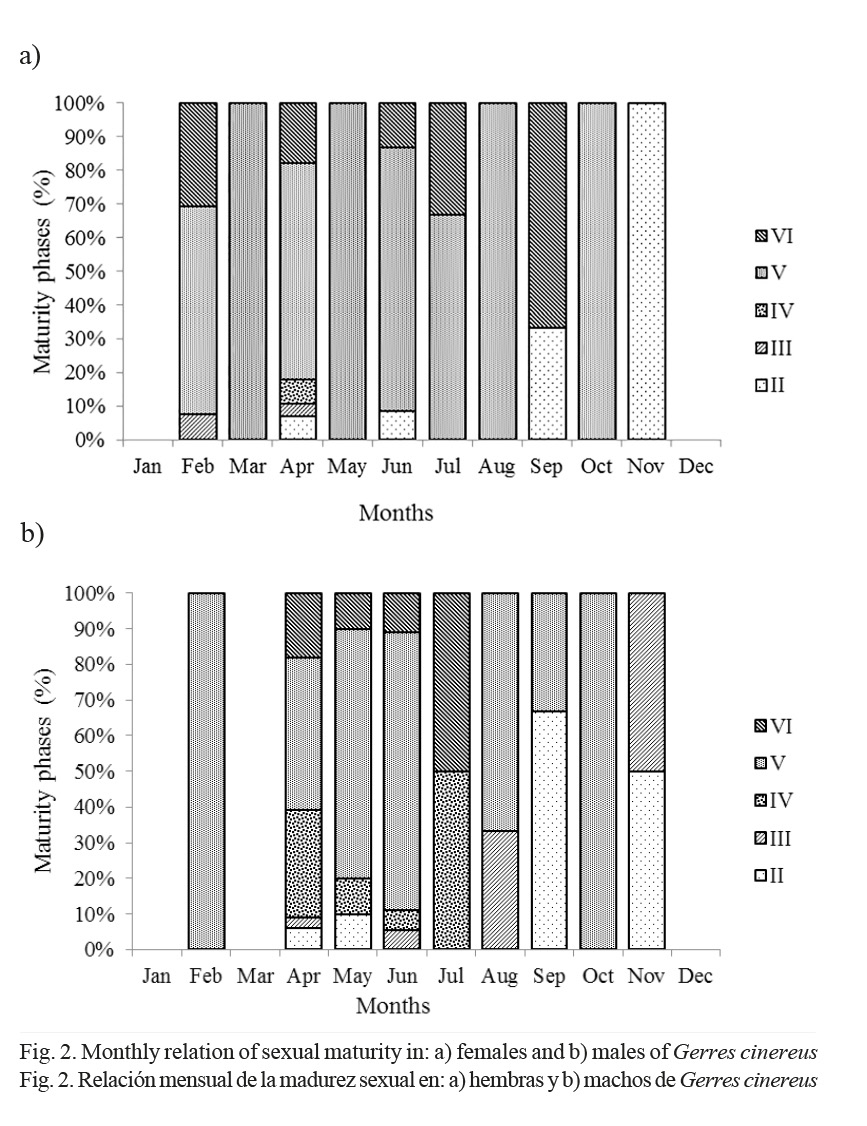
Monthly variations of relative frequency for gonad maturity phases show that phase II or immature females and males prevail in September and November (Fig. 2a, b), while phase IV, mature, was observed from April to July. Phase V or spawning stage was observed in females from February to May and August to October. Males were in phase V from February to June and August to November. Phase VI, post-spawning, was observed in females in February, April, June and September, while males were from April to July (Fig. 2a, b).
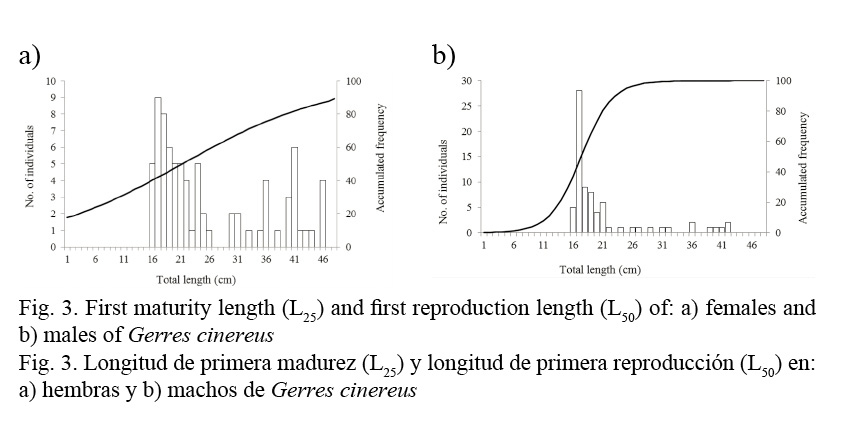
Length at first maturity was L25 = 16.50 cm in females (Fig. 3a) and L25 = 15.80 cm in males (Fig. 3b), corresponding to less than one year of age. First reproduction length was L50 = 20.20 cm in females and L50 = 16.40 cm in males (Fig. 3), which corresponds to one year of age.
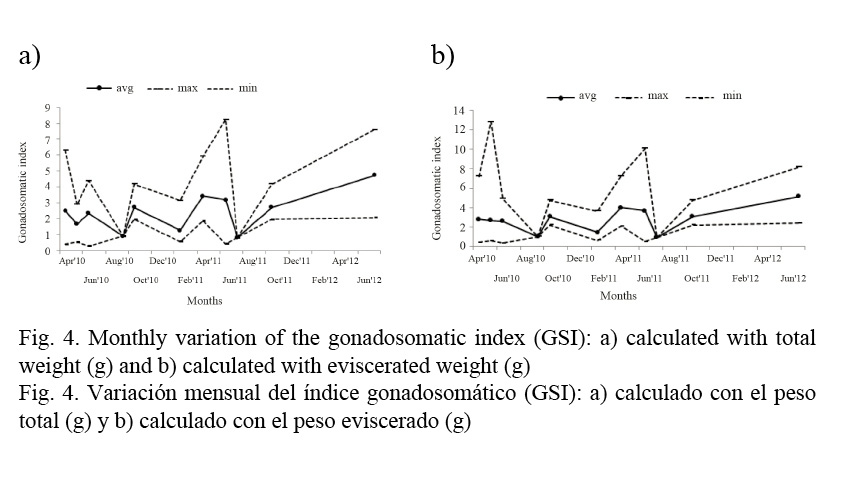
The gonadosomatic index (GSI) reaches the highest values in June for total length and eviscerated weight (Fig. 4a, b) seconded by a spawning period during October. GSI values decrease during August and February.
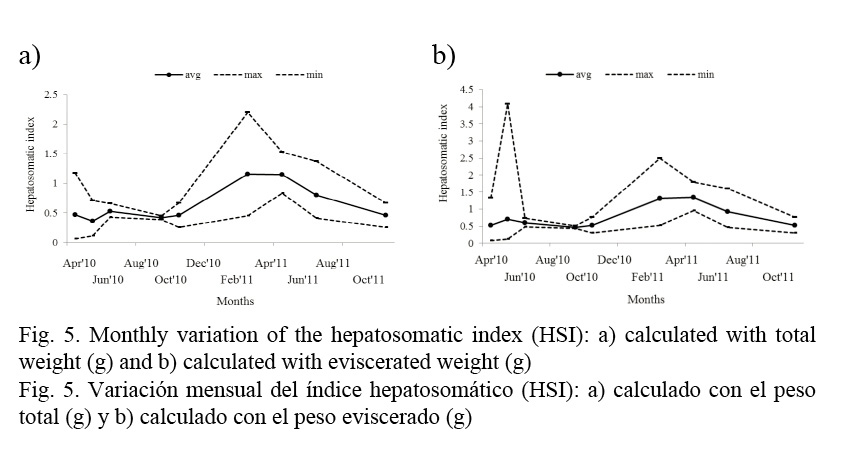
The allometric relationship of the hepatosomatic index (HSI) obtained in the present study was LW = 2.00·10−6 · TL4.213 (r2 = 0.895). The allometric index b indicates that, in terms of length, the liver weight is higher than a cubic proportion, which results in a positive allometric growth of the fish and an increase of its fatty reserves as it ages. HSI variations are shown in Figures 5a and b; maximum values are observed in February and lower values in September.
Variations in the stomach repletion index (Fig. 6a, b) showed higher values during April and May, preceding the spawning season; lower values are observed from October to February and June to October.
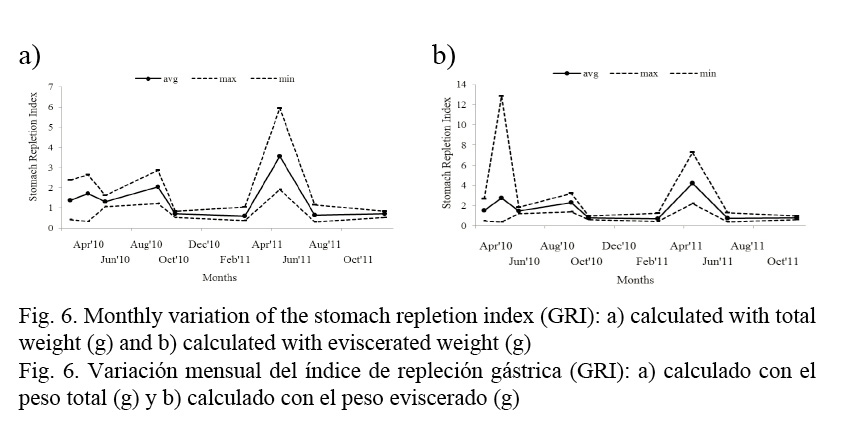
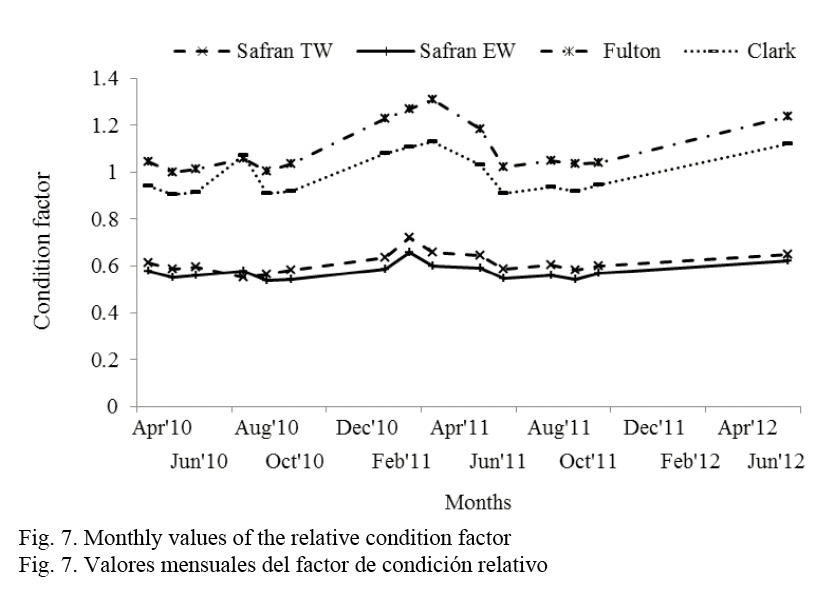
Figure 7 shows the values of the condition factor; the highest values are obtained in February and September.
DISCUSSION
The highest length growth rates of G. cinereus (calculated by Espino-Barr et al. 2014; 2015) are in groups one and two years of age, after which length growth rate starts to diminish and total weight and gonad weight start to rise, as well as the fatty reserve index. Therefore, two fundamental periods were considered in the life cycle of G. cinereus: first, when most of the energy obtained through food is used to increment length (to avoid depredation and interspecific competence) and second, when this energy is oriented to form the sexual products (Fig. 8) (Espino-Barr et al. 2008, Cabral-Solís et al. 2010).
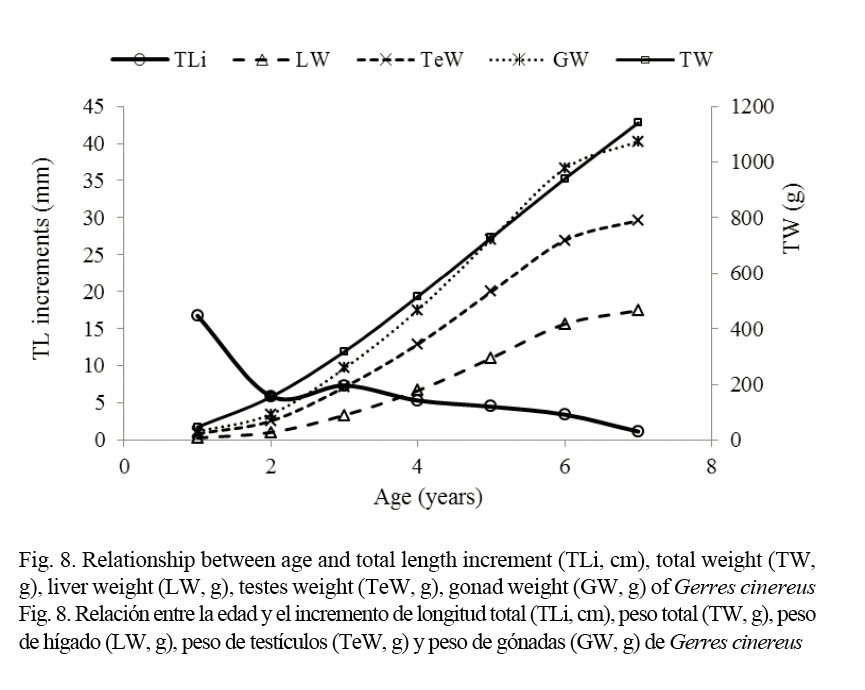
Sexual proportion was 1:1.024 female: male, similar to 1.2:1 female: male found in Eugerres mexicanus in the Usumacinta river in Mexico (Valdez-Zenil et al. 2013). Higher values were found for Eugerres plumieri in the Maracaibo lake, Venezuela, 1.77:1 female: male (Ferrer-Montaño, 2009).
Although the presence of mature G. cinereus happens throughout the entire year, massive spawning occurs in June followed by a second period of massive spawning observed in October. Table 2 shows spawning periods for different species of the Gerreidae family. Except for Eugerres mexicanus, the other species show the massive spawning period at the end of spring and in the summer. In the case of G. cinereus from the coast of Cuba (García-Cagide et al. 1999), its main spawning peak was at the same period as in the Central Mexican Pacific coast.
According to its first sexual maturity length, Gerres oyena from Japan is a precocious species (compared to others of the same family). It matures at lengths of 8.97 cm (females) and 8.14 cm (males), followed by Gerres equulus also from Japan, with first sexual maturity length values of 14.1 cm in females. G. cinereus presented intermediate values, compared to other species: 16.5 cm females and 15.8 cm males. Nevertheless, the minimum spawning age found in this study was before the first year of life. In Cuba, G. cinereus matures at 20 cm TL (García-Cagide et al. 1999), a higher value than in the Pacific coast. Higher values were found in Eugerres mexicanus of the Usumacinta River: 20.5 cm in females and 17.3 cm in males (Valdez-Zenil et al. 2013), and in E. plumieri of Lake Maracaibo, Venezuela, at 18 cm for females and 17 cm for males (Ferrer-Montaño, 2009).
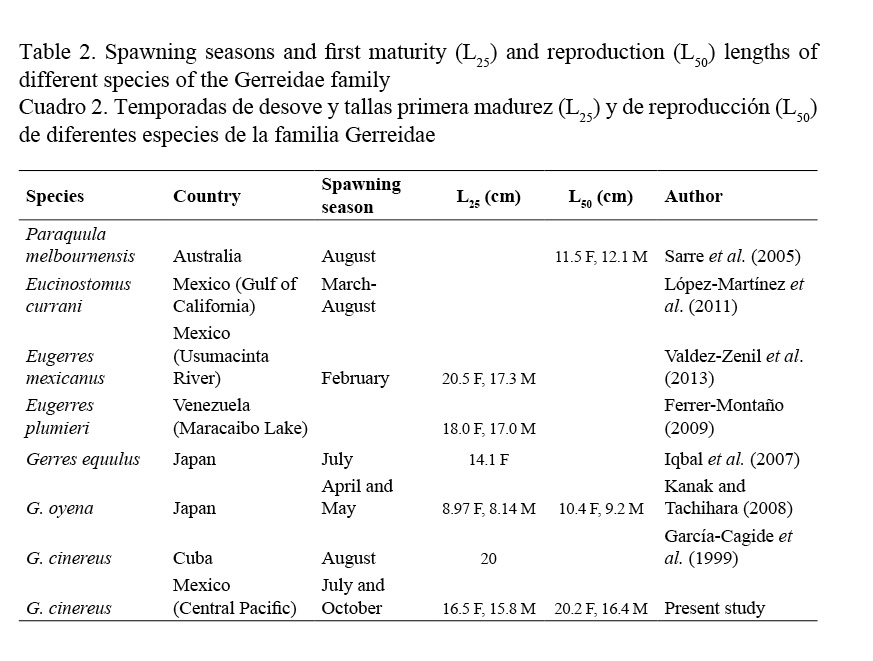
The first G. cinereus reproduction sizes were the highest at 20.2 cm in females and 16.4 cm in males, corresponding to ages 1 and almost 2. Comparately, G. oyena in Japan was smaller: 10.4 cm in females and 9.2 cm in males (Kanak & Tachihara, 2008); and also in Australia (Sarre et al. 2005), Paraquula melbournensis was 11.5 cm in females and 12.1 cm in males.
The hepatosomatic index obtained in this study was b= 4.213 (r2 = 0.895), which indicates a positive allometric growth, since fish increase their fatty reserves as they grow older. Monthly values showed that the liver accelerates its activity of reserving fatty acids during the periods before spawning; therefore, their weight increases considerably. The highest activity of fatty acid reserves is in February and starts to decrease in June and August, as well as in October during the spawning period.
Similarly, in the Island of Okinawa in south Japan, hepatosomatic index in Gerres oyena decreases during the maximum gonadic development that occurs in April and May for both sexes (Kanak & Tachihara, 2008).
The most active feeding seasons are during the periods of time prior to spawning, in spring; the most active months are April and May; the stomach repletion index decreases during the spawning months, and declines significantly after the second massive spawning period, that is October.
The stomach repletion index decreases in winter and increases in spring (Fig. 6a, b). This cycle is associated with environmental conditions and their effect on the food supply (Claro et al. 2001). The reproduction cycle is closely related to the availability of enough food for offspring (Canan et al. 2011).
Condition factor reaches the maximum values during February with either model: Fulton (1902); Clark (1928) and Safran (1992), and decreases during spawning seasons, that is, during June to September; and November-December, after the massive spawning of October.
Similarly, Fulton’s condition factor of G. equulus from Japan reached its maximum values during the spawning season from June to September (Iqbal & Suzuki, 2009). In the Island of Okinawa (south Japan), this condition factor decreases during the gonadic development in G. oyena (Kanak & Tachihara, 2008).
Recruitment length of G. cinereus to the fishing area was at 14 cm TL and occurred in April, which means that this species spends 9 months as part of the plankton system and other marine strata before becoming part of the adult population. The same happens to Eugerres plumieri, which recruits to the adult population during April (Ferrer-Montaño, 2009).
No evidence of migratory movement was found in G. cinereus as to spawn in the sea. Adults were found both inside the lagoons and out in the sea, contrary to what has been reported for the species of G. melanopterus in the Bay of Guaratuba in Paraná, Brazil, where adults are known to migrate out to sea to spawn during summer and come back to the bay during fall and winter (Chaves & De Castro, 2001). In addition, G. rappi, G. acinaces and G. filamentosus were described as going in estuaries when reaching 10 mm standard length and stay there till they reach sexual maturity between 70 and 110 mm, after which females and males leave the estuary before eggs mature (Cyrus & Blaber, 2006).
A fishing ban has not been established in Mexico for the Gerreidae family. In the case of Eucinostomus currani from the Gulf of California, it is protected during the shrimp ban, because it is caught as bycatch (López-Martínez et al. 2011).
As García-Cagide et al. (1983) summarizes, the Gerreidae family is basically composed of tropical species of fish that reach sexual maturity at early ages. Sexual products developed rapidly and can be observed all months of the year, as opposed to species of template and cold climates that reach sexual maturity at an advanced age, take a long period of time to form sexual products and have a very specific spawning season.
These observations must be reinforced with research on other members of the Gerreidae family because these can represent adaptive as well as evolutionary advantages of this family in relation to other fish groups (García-Cagide et al. 1994).
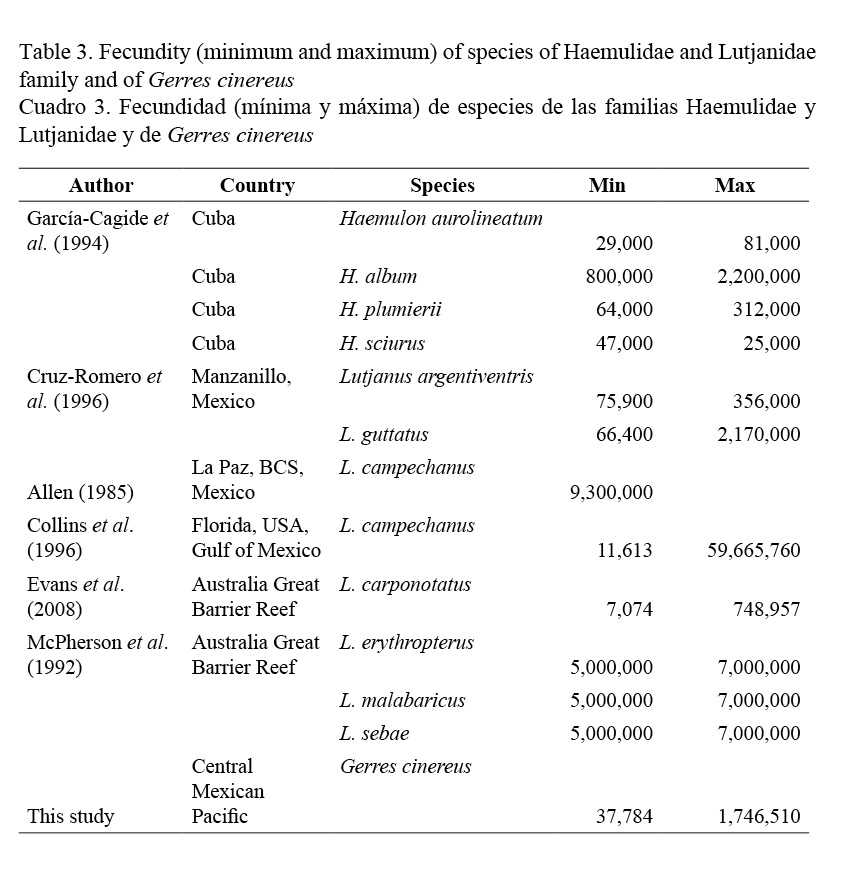
Table 3 shows fecundity values in species of three phylogenetic close families (Nelson et al. 2004; Nelson, 2006): Haemulidae, Lutjanidae and Gerreidae, and how G. cinereus occupies an intermediate position compared to the species of other families. G. cinereus has a higher fecundity value than Haemulon aerolineatum, H. plumieri, H sciurus, Lutjanus argentiventris and L. carponotatus, but lower than Haemulon album, Lutjanus guttatus, L. campechanus, L. erythropterus, L. malabaricus and L. sebae.

Reproduction of Gerres cinereus (Percoidei: Gerreidae) off the Mexican Pacific coast por Revista Ciencias Marinas y Costeras se distribuye bajo una Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Costa Rica License.
Basada en una obra en http://www.revistas.una.ac.cr/index.php/revmar.
Permisos que vayan más allá de lo cubierto por esta licencia pueden encontrarse en revmar@una.cr.
BIBLIOGRAPHY
Aboussouan, A. & Lahaye, J. (1979). Les potentialités des populations ichthyologiwues. Fécondité et echthyoplancton. Cybium, 3e sér., 6, 29-46.
Allen, G. R. (1985). FAO Species catalogue. Snappers of the World. An annotated and illustrated catalogue of lutjanid species known to date. FAO Fish. Synop., 6(125), 1-208.
Allen, G. R. & Robertson, D. R. (1994). Peces del Pacífico Oriental Tropical. México: CONABIO, Agrupación Sierra Madre y CEMEX.
Bussing, W. A. (1995). Gerreidae: mojarras. In W. Fischer, F. Krupp, W. Schneides, C. Sommer, K. E. Carpenter & U. H. Niem (Eds.), Guía FAO para identificación de especies para fines de la pesca. Pacífico Centro-Oriental Vol II y III (pp. 1114-1128). Roma, Italia: FAO.
Cabral-Solís, E. G., Gallardo-Cabello, M., Espino-Barr, E. & Ibáñez-Aguirre, A. L. (2010). Reproduction of Mugil curema (Pisces: Mugilidae) from the Cuyutlan Lagoon, in the Pacific coast of Mexico. AIA, 14(3), 19-32.
Canan, B., Rodrigues Pessoa, E. K., Volpato, G. L., Araújo, A. & Chellappa, S. (2011). Feeding and reproductive dynamics of the Damselfish, Stegastes fuscus in the coastal reefs of Northeastern Brazil. An. Biol. J., 2(3), 113-126.
Chaves, P. C. & De Castro, M. (2001). Nota complementar sobre os hábitos de Gerres melanopterus (Teleostei, Gerreidae) na Baia de Guaratuba, Paraná, Brasil (25° 51’ S, 48° 39’ W). Rev. Bras. Zool., 18(1), 255-259. doi: 10.1590/S0101-81752001000100028
Clark, F. (1928). The weigth-length relationship of the Californian sardine (Sardina coerulea) at San Pedro. Fish. Bull. U.S., 12, 22-44.
Claro, R., Lindeman, K. C. & Parenti, L. R. (2001). Ecology of the marine fish of Cuba. Washington, USA: Smithsonian Institution Press.
Collins, L. A., Johnson, A. G. & Keim, C. P. (1996). Spawning and annual fecundity of the red snapper (Lutjanus campechanus) from the northeastern Gulf of Mexico. In F. Arreguín-Sánchez, J. L. Munro, M. C. Balgos & D. Pauly (Eds.), Biology, fisheries and culture of tropical groupers and snappers (pp. 174-188). Manila, Philippines: ICLARM Conf. Proc. 48.
Cruz-Romero, M., Chávez, E. A., Espino-Barr, E. & García-Boa, A. (1996). Assessment of a snapper complex (Lutjanus spp.) of the Eastern Tropical Pacific. In F. Arreguín-Sánchez, J. L. Munro, M. C. Balgos & D. Pauly (Eds.), Biology, fisheries and culture of tropical groupers and snappers (pp. 324-330). Manila, Philippines: ICLARM Conf. Proc. 48.
Cyrus, D. P. & Blaber, S. J. M. (2006). The reproductive biology of Gerres in Natal estuaries. J. Fish Biol., 24(5), 491-504. doi: 10.1111/j.1095-8649.1984.tb04820.x
Espino-Barr, E., González Vega, A., Santana Hernández, H. & González Vega, H. (2008). Manual de biología pesquera. Tepic, Nayarit, México: Universidad Autónoma de Nayarit.
Espino-Barr, E., Nava Ortega, R. A., Gallardo-Cabello, M., Cabral-Solís, E. G., Puente-Gómez, M. & García-Boa, A. (2012). Aspects of Scomberomorus sierra fishery from the coast of Colima, Mexico. Cienc. Pesq., 20(1), 77-88.
Espino-Barr, E., Gallardo-Cabello, M., Cabral-Solís, E. G., Puente-Gómez, M. & García-Boa, A. (2014). Growth of the Yellowfin Mojarra Gerres cinereus off the Pacific Coast of Mexico. J. Fish. Aq. Sci., 9(1), 14-23. doi: 10.3923/jfas.2014.14.23.
Espino-Barr, E., Gallardo-Cabello, M., Cabral-Solís, E. G., García-Boa, A. & Puente-Gómez, M. (2015). Analysis of the otoliths sagitta, asteriscus and lapillus of Yellowfin Mojarra Gerres cinereus (Perciformes: Gerreidae) in the coast of Colima and Jalisco, Mexico. O. J. OCS 2(1), 18-33. doi: 10.15764/OCS.2015.01002
Evans, R. D., Russ, G. R. & Kritzer, J. P. (2008). Batch fecundity of Lutjanus carponotatus (Lutjanidae) and implications of no-take marine reserves on the Great Barrier Reef, Australia. Coral Reefs, 27(1), 179-189. doi: 10.1007/s00338-007-0309-8
Ferrer-Montaño, O. J. (2009). Catch dynamics, growth, and reproduction of striped Mojarra Eugerres plumieri in Lake Maracaibo, Venezuela. Ciencia, 17(2), 141-150.
Fulton, T. (1902). Rates of growth of sea-fishes. Sci. Invest. Fish. Div. Scot. Rept., 1, 21-3720.
Gaertner, D. & Laloe, F. (1986). Etudebiometrique de la talle a’premier maturité sexualle de Geryonmaritae, Maning et Holthuis, 1981 de Senegal. Oceanol. Acta, 9(4), 479-487.
Gallardo-Cabello, M., Espino-Barr, E., García-Boa, A., Cabral-Solís, E. G. & Puente-Gómez, M. (2007). Study of the growth of the green jack Caranx caballus Günther 1868, in the coast of Colima, Mexico. J. Fish. Aq. Sci., 2(2), 131-139.
García-Cagide, A., Claro, R. & Koshelev, B. V. (1983). Peculiaridades de los ciclos reproductivos de los peces de diferentes latitudes. Habana, Cuba: Academia de Ciencias.
García-Cagide, A., Claro, R. & Koshelev, B. V. (1994). Reproducción. In R. Claro (Ed.) Ecología de los peces marinos de Cuba, (pp. 187-262). Chetumal, Q. R. México: Inst. Oceanol. Acad. Crinc. Cuba and Cent. Invest. Quintana Roo (CIQRO).
García-Cagide, A., Claro, R. & García-Arteaga, J. P. (1999). Biología del jocú, Lutjanus jocu (Pisces: Lutjanidae) en las zonas NE y SW de la plataforma cubana, I. Distribución, hábitat, reproducción y dinámica de los indicadores morfofisiológicos. Rev. Invest. Mar., 20(1-3), 22-29.
Holden, M. J. & Raitt, D. F. S. (1975). Manual de Ciencia Pesquera. Métodos para investigar los recursos y su aplicación. México: ONU/FAO.
Iqbal, K. M., Ohtomi, J. & Suzuki, H. (2007). Reproductive biology of the Japanese silver-biddy, Gerres equulus, in western Kyushu, Japan. Fish. Res., 83(2-3), 145-150. doi: 10.1016/j.fishres.2006.09.019
Iqbal, K. M. & Suzuki, H. (2009). Length-weight relationships and condition factor of the Japanese silver-biddy, Gerres equulus, in the Yatsushiro Sea, western Kyushu, Japan. J. App. Ichth., 25(2), 219-222. doi: 10.1111/j.1439-0426.2008.01207.x
Kanak, M. K. & Tachihara, K. (2008). Reproductive biology of common silver biddy Gerres oyena in Okinawa Island of southern Japan. Fish. Sci., 74(2), 265-275. doi: 10.1111/j.1444-2906.2008.01521.x
López-Martínez, J., Rodríguez-Romero, J., Hernández-Saavedra, N. Y. & Herrera-Valdivia, E. (2011). Population parameters of the Pacific flagfin mojarra Eucinostomus currani (Perciformes: Gerreidae) captured by shrimp trawling fishery in the Gulf of California. Rev. Biol. Trop., 59(2), 887-897.
McPherson, G. R., Squire, L. & O’Brien, J. (1992). Reproduction of three dominant Lutjanus species of the Great Barrier Reef inter-reef fishery. Asian Fish. Sci., 5(1), 15-24.
Nelson, J. S. (2006). Fishes of the World. Alberta, Canada: John Wiley & Sons, Inc.
Nelson, J. S., Crossman, E. J., Espinosa-Pérez, H., Findley, L. T., Gilbert, C. R., Lea, R. N. & Williams, J. D. (2004). Common and scientific names of fishes from the United States, Canada and Mexico. California, EE.UU.: American Fisheries Society.
Rodríguez-Gutiérrez, M. (1992). Técnicas de evaluación cuantitativa de la madurez gonádica en peces. México: AGT Ed.
Safran, P. (1992). Theoretical analysis of the weight-length relationship in fish juveniles. Mar. Biol., 112, 545-551. doi: 10.1007/BF00346171
SAGARPA. (2012). Anuario estadístico de pesca 2009. México: Comisión Nacional de Acuacultura y Pesca, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación.
Sarre, G. A., Hyndes, G. A. & Potter, I. C. (2005). Habitat, reproductive biology and size composition of Parequula melbournensis, a Gerreid with a temperate distribution. J. Fish Biol., 50(2), 341-357.
Simpson, A. C. (1951). The fecundity of the plaice. Fish. Invest., 18(5), 1-27.
Sokolov, V. & Wong, R. M. I. (1973). Programa general para la investigación de los peces pelágicos del Golfo de California. PNUD/FAO. México. CEPM, 3, 1-51.
Sparre, P. & Venema, S. C. (1995). Introducción a la evaluación de recursos pesqueros tropicales. Manual. Roma, Italia: FAO.
Valdez-Zenil, J., Rodiles-Hernández, R., González-Acosta, A. F., Mendoza-Carranza, M. & Barba Macías, E. (2013). Length-weight and length-length relationships, gonadosomatic indices and size at first maturity of Eugerres mexicanus (Steindachner, 1863) (Percoidei: Gerreidae) from the Usumacinta River, Mexico. J. App. Ichth., 30(1), 210-220. doi:10.1111/jai.12267