Rev. Mar. Cost. ISSN 1659-455X. Vol. 8 (2): 29-45, Julio-Diciembre 2016.
DOI: http://dx.doi.org/10.15359/revmar.8-2.2
Seasonal changes of phytoplankton in the Paquera-Tambor Marine Area for Responsible Fishing, Gulf of Nicoya, Costa Rica
Cambios estacionales del fitoplancton en el área marina de pesca responsable de Paquera-Tambor, Golfo de Nicoya, Costa Rica
Andrea García-Rojas1* & Hannia Vega Bolaños1
1 Juan Bertoglia Richards Marine Biology Station, School of Biological Sciences, Universidad Nacional, Costa Rica. andrea.garcia.rojas@una.cr*; hannia.vega.bolaños@una.cr
Recibido: 27 de julio de 2015
Corregido: 3 de junio de 2016
Aceptado: 15 de junio de 2016
ABSTRACT
The implementation of Marine Areas for Responsible Fishing (MARF) is a tool for fisheries management; therefore, baseline studies play an important role in understanding the ecological dynamics from the bases of the food web in the MARF. The aim of this study was to identify the abundance of the phytoplankton communities associated with the MARF to determine the seasonal changes between abiotic variables and phytoplankton in the Paquera-Tambor MARF, Gulf of Nicoya, Costa Rica. Monthly sampling (September 2013 to August 2014) was performed for physical-chemical factors and phytoplankton. The data showed a temporal variation of both environmental factors and the phytoplankton community. The most representative microalgae were diatoms and dinoflagellates with a richness of 51 and 32 species, respectively, where the presence of some algal bloom forming species such as Cochlodinium catenatum was highlighted, with a concentration of 5.85x104 cells L-1. Regarding diatoms and parameters such as Secchi disk depth (r = -0.558) and the percentage of oxygen saturation (r = -0.490), a negative correlation was found due to climate variability in the area. Zooplanktonic tintinnids were identified and showed a positive correlation with diatoms (r = 0.433). A fundamental ecosystem dynamic was evident for the trophic development of the Tambor-Paquera-MARF, which underpins the importance of the fishing zone and reflects the relevance of continued biotic and abiotic monitoring for the area.
Keywords: Microalgae, seasonal changes, Marine Areas for Responsible Fishing, Gulf of Nicoya, Costa Rica.
RESUMEN
La implementación de Áreas Marinas de Pesca Responsable (AMPR) es una herramienta para el ordenamiento pesquero, por ello, los estudios de línea base juegan un rol importante para comprender la dinámica ecológica desde las bases de la red trófica en las AMPR. El objetivo de este trabajo fue caracterizar la abundancia de los grupos taxonómicos del fitoplancton en el AMPR-Paquera-Tambor, para la determinación de cambios estacionales entre variables abióticas y el fitoplancton. Se realizó un muestreo mensual (de septiembre-2013 a agosto-2014) para la toma de muestras de factores fisicoquímicos, así como de microalgas planctónicas. Los datos evidenciaron una variación temporal tanto de los factores ambientales como del fitoplancton. Las microalgas más representativas fueron las diatomeas y los dinoflagelados con una riqueza de 51 y 32 especies, respectivamente, donde se resalta la presencia de algunas especies productoras de florecimientos algales como Cochlodinium catenatum con una concentración de 5.85x104cél L-1. Con respecto a las diatomeas y los parámetros, como la profundidad del disco Secchi (r = -0.558) y el porcentaje de saturación de oxígeno (r = -0.490), se reflejó una correlación negativa debido a la variabilidad climática de la zona. Se identificaron organismos del grupo zooplanctónico de los tintínidos, los cuales presentaron una correlación positiva con las diatomeas (r = 0.433). Se evidenció una dinámica ecosistémica fundamental para el desarrollo trófico del AMPR-Paquera-Tambor, que fundamenta la importancia pesquera de la zona y refleja la relevancia de continuar con un monitoreo biótico y abiótico para la zona.
Palabras claves: Microalgas, cambios estacionales, áreas marinas de pesca responsable, Golfo de Nicoya, Costa Rica.
INTRODUCTION
In Costa Rica, overfishing and the lack of planning in the use of coastal resources has promoted the implementation of Marine Areas for Responsible Fishing (MARF), which are marine areas demarcated and managed by coastal communities together with the relevant authorities in order to regulate fishing activities with a better use of resources in the long term (MAG, 2009). MARFs provide some advantages for fisheries management. For instance, they reduce conflict in the use of fishing gear harmful to marine biodiversity that affect the abundance of organisms. Fishing efforts are reduced in recruitment areas, which leads to conservation of sites and resources. There is a better collection of fisheries data which helps improve policies that directly affect the sector. Mainly, products are guaranteed to come from a sustainably exploited area that combines the human development of the social groups involved (OCEANA, 2013; Hoff et al. 2015).
Given the advantages provided by this fishery regulation, there are a number of provisions that must be met to designate an area as a MARF, including baseline studies, which represent a fundamental tool to learn about the biodiversity and environmental state of the ecosystem to be managed. As a result, the analysis of the phytoplankton community is important, since this group of organisms represents the plant material of marine plankton, and they are the primary producers of the aquatic ecosystems, which bears ecological importance since this is the basis of the marine food chain (Valiela, 1995; Rochelle-Newall et al. 2011). This group is made up of a wide variety of taxa, including the Cyanophyta, Prochlorophyta, Chlorophyta, Euglenophyta, Dinophyta, Haptophyta, Cryptophyta, and Chromophyta (Bacillariophyceae, Chrysophyceae, Raphidophyte, and Prymnesiophyceae), with diatoms and dinoflagellates being the most diverse groups (Dawes, 1991; Valiela, 1995; Jeffrey et al. 1997; Knox, 2000; Mann, 2000; MacIntery et al. 2000; Nybakken, 2001; Throndsen et al. 2007; Hoppenrath et al. 2009; Simon et al. 2009; Widdicombe et al. 2010).
Phytoplankton, due to their short life cycles, respond quickly to environmental changes, and their qualitative and quantitative composition allows an estimation of water quality. The development of these organisms is controlled by biological, physical and chemical processes (Hu et al. 2011); therefore, the information that they may give as bioindicators should be interpreted with other physical and chemical data. In addition, the rapid response of the phytoplankton community to changes induced by human activity (introduction of nutrients, organic matter, or pollutants) currently makes them a key element in the evaluation of the quality of seawater (Domingues et al. 2008; Spatharis & Tsirtsis, 2010).
Similarly, interspecific relationships in communities affect the stability of food webs, which have been evaluated in order to analyze their effects on ecosystems and their relationship with trophic chains (Li et al. 2009); for instance, the relationship between phytoplankton and cod production has been studied, which shows that primary productivity determines the load capacity of systems (Steingrund & Gaard, 2005; Hansen et al. 2005) and, therefore, the production of commercial species.
The aim of this study was to characterize the abundance of the taxa of the phytoplankton in the Paquera-Tambor Marine Area for Responsible Fishing to determine seasonal changes of microalgae, influenced by abiotic variables.
MATERIALS AND METHODS
Study area
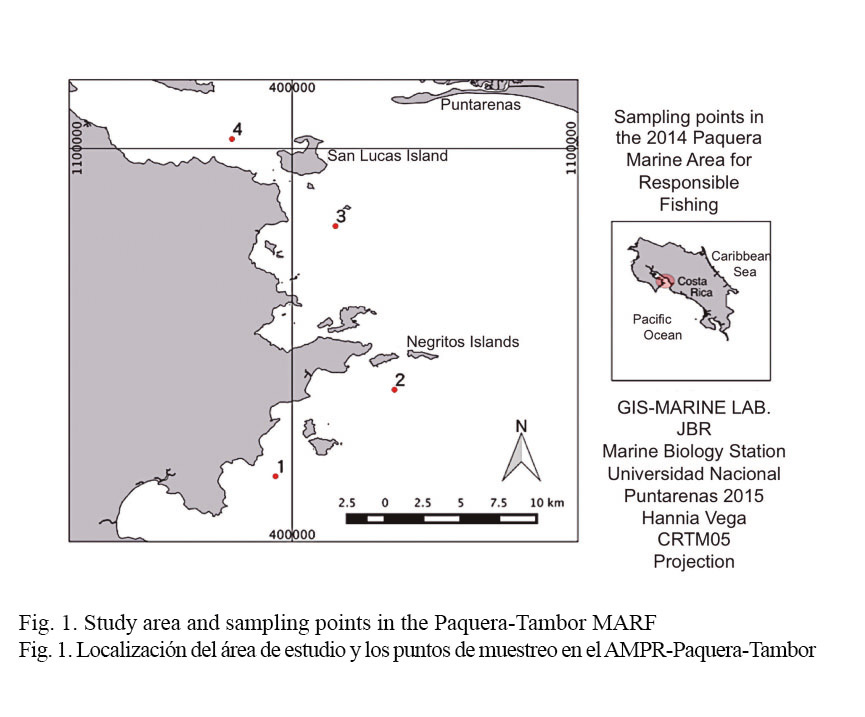
This study was conducted on the outer part of the Gulf of Nicoya (GN), Costa Rica, specifically in the Paquera-Tambor Marine Area for Responsible Fishing (Paquera-Tambor-MARF). This area is characterized for having the greatest depths within the GN (25 to 100 m) and a strong influence by oceanic water masses (Brenes & León, 1995).
Four sampling stations were located in the Paquera-Tambor MARF, taking into account bathymetric, geographic and biological characteristics of the area. Stations 1 and 2 were located in the outermost zone in the Paquera-Tambor MARF, the first station in front of the Tambor Beach area, with depths close to 45 m, and the second station between Tortuga Island and the Negritos Islands, since the latter constitute a natural barrier dividing the MARF. Sampling station 3 was located in front the sanitary landfill (El Relleno) in Paquera, since it is an important area for the reproduction and growth of the spotted snapper Lutjanus guttatus (Steindachner, 1869), a fish species important for the area (Araya et al. 2007). Station 4 was located between San Lucas Island and Naranjo Beach, in the shallowest and innermost part of the sampling area (Fig. 1).
Collecting data and water samples:
Monthly water and environmental data samples were taken from September 2013 to August 2014 for phytoplankton analysis. Temperature, salinity, total dissolved solids concentration, and percentage of dissolved oxygen saturation were measured with a YSI 556 MPS multiparameter in-situ at each of the sampling stations, as well as the Secchi disk depth.
In order to determine phytoplankton abundance, water samples were collected with a 6L Niskin bottle at a 2m depth. Water samples were stored in 500 ml plastic bottles (previously washed with water from the site), and a 5 ml Lugol solution was added to preserve the microalgae.
Identification and counts of phytoplankton:
Phytoplankton organisms were studied using 1 ml Sedgewick Rafter counting chambers and a Nikon’s Eclipse E600 light microscope, for which a 1 ml aliquot of the sample was placed directly on the cell count (the sample was not concentrated). Phytoplankton were counted and identified observing the entire bottom of the counting chamber with 10x and 20x objectives, depending on the dimensions of the organisms. Organisms were identified up to the lowest possible taxonomic level and counts were done making groups of cells according to the main phytoplankton groups identified.
The studies by Cupp (1937), Sournia (1986), Ricard (1987), Chrétiennot-Dinet (1990), Round et al. (1990), Tomas (1997), Horner (2002), and Ojeda (2006) were used for the identification of microalgae.
Data analysis:
In order to determine the normality of data obtained, a Levene’s test was used, and, in cases where data did not follow a normal curve, data was transformed using the log(x+1) function.
In order to determine significant temporal differences between biological and abiotic parameters, a one-way ANOVA test was used, and the significance of each variable was confirmed with a multiple comparison Student-Newman-Keuls (S-N-K) post-hoc test. The relationship of the different phytoplankton groups identified with respect to the physical and chemical factors was determined using the Pearson correlation. The ANOVA, correlation, and normality analyses were conducted using the SPSS 17.0 statistical program (SPSS, 2008).
RESULTS
Determining the temporal variation between the sampling months for the physical-chemical variables and the phytoplankton groups showed significant differences (P <0.05) for parameters such as temperature (P <0.001), salinity (P <0.001), total solids (P <0.001) and percentage of oxygen saturation (P <0.001), as well as for dinoflagellates (P <0.048) and diatoms (P <0.016) (Table 1). Since sampling began in the rainy season, temperature tended to increase throughout the study period, with a minimum value of 27°C in October and a maximum value of 30°C in April. For variables such as salinity, total solids concentration and percent of dissolved oxygen saturation, the period with the highest values was mainly the dry season, with values equal to 33.57PSU, 33.36 mg L-1 and 126.7%, respectively. The Secchi disk depth ranged between 0.7m and 14.6m, with a strong variability during the sampling period (Figs. 2 and 3).
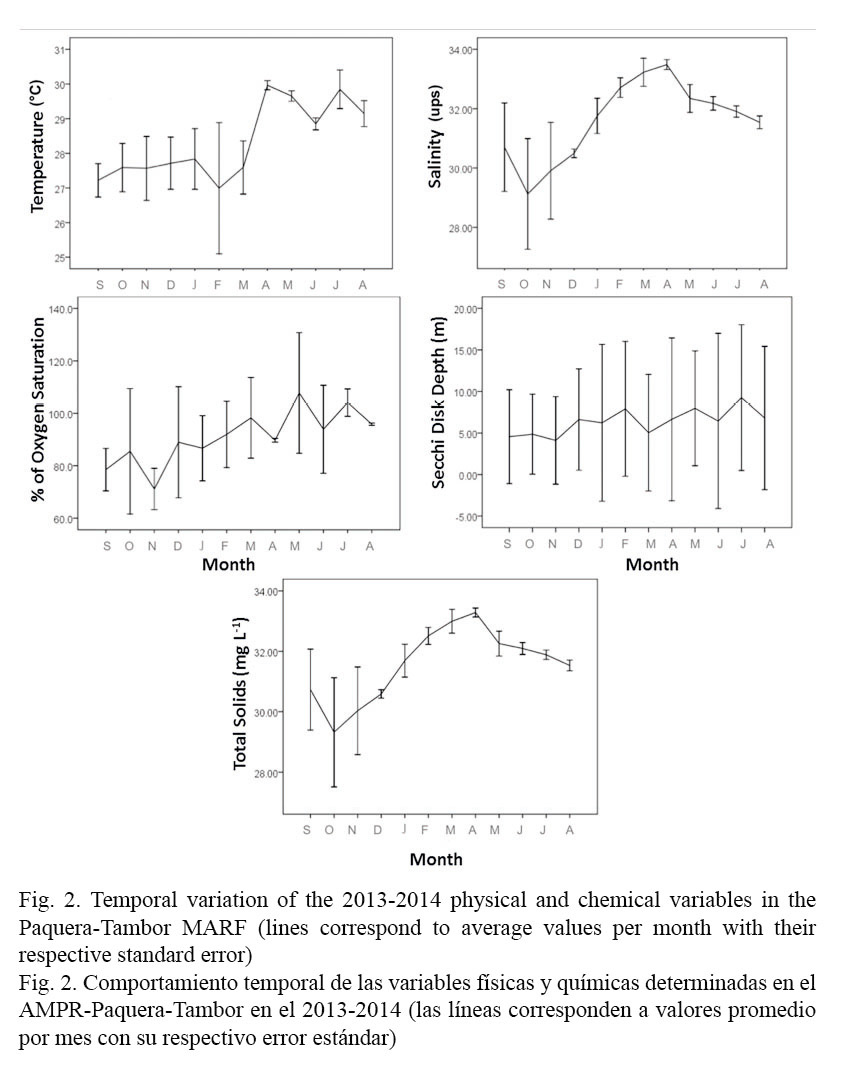

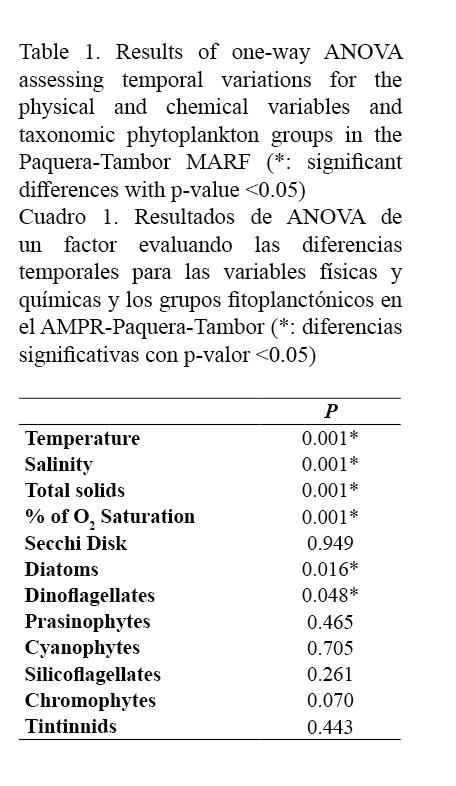
Regarding the micro-algae community, diatoms and dinoflagellates were the most representative groups throughout the sampling period, followed by the silicoflagellates, with values of 1.3x104 ± 2.3x103 cells L-1, 5.9x104 ± 9.1x103 cells L-1 and 2.9x102 ± 84 cells L-1, respectively. It is worth highlighting that dinoflagellates showed algal blooms associated with the harmful species, Cochlodinium catenatum, which exhibited a concentration of 5.85x104 ± 3x104 cells L-1 during October 2013. Other phytoplankton groups such as the prasinophytes (125 ± 125 cells L-1), cyanophytes (84 ± 40 cells L-1) and chromophytes (94 ± 60 cells L-1) had a sporadic presence and relatively low concentrations in comparison with the dominant groups. In addition, the zooplanktonic group tintinnids was present throughout the study period, although in low concentrations (ranging between 1x103 and 2.5x103 cells L-1) (Fig. 3).
The same trend seen in the abundance was observed in species richness, with diatoms and dinoflagellates showing 51 and 32 species, respectively. For the remaining phytoplankton groups identified, abundance of species ranged between one and four specimens, while eight species were observed for tintinnids (Table 2).
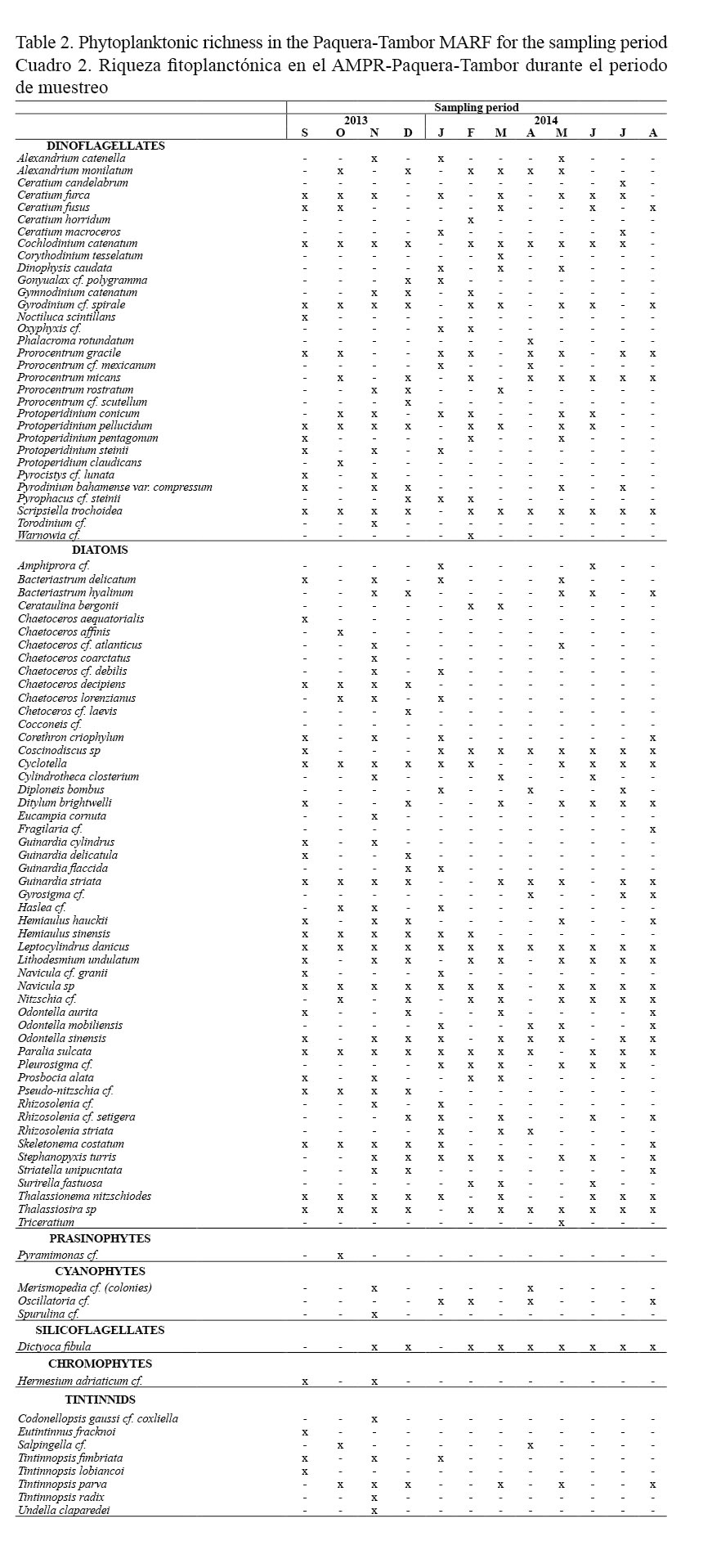
Within the dinoflagellates, the presence of the following algal bloom forming organisms was highlighted: dinoflagellates Cochlodinium catenatum (Okamura, 1916), Pyrodinium bahamense var. compressum (Böhm, Steidinger, Tester & Taylor, 1980), Alexandrium monilatum (Balech, 1995), and diatoms Pseudo-nitzschia spp. However, during the study period, only the dinoflagellates A. monilatum and C. catenatum generated an algal bloom with water discoloration and production of mucous substances.
The most dominant phytoplankton species were the diatoms Cyclothella spp., Guinardia striata (Hasle, 1996), Leptocylindrus danicus (Cleve, 1889), Navicula spp., Nitzschia spp., and Thalassiosira spp., with frequency percentages in the samples greater than 50%. Within the same frequency range, the dinoflagellates Gyrodinium spirale (Kofoid and Swezy, 1921), Scripsiella trochoidea (Loeblich III, 1976) and Cochlodinium catenatum were observed.
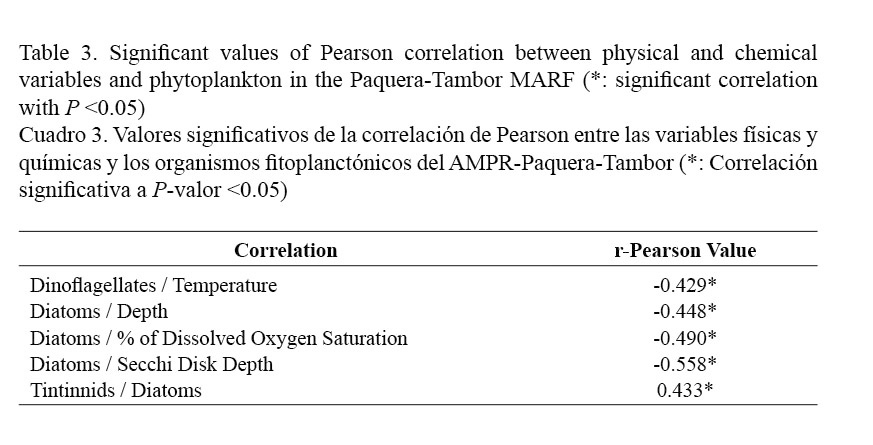
When determining the correlation between environmental variables and phytoplankton, negative relationships were observed between diatoms and some variables such as depth (r = -0.448), percentage of dissolved oxygen saturation (r = -0.490) and Secchi disk depth (r = -0.558), as well as between dinoflagellates and water temperature (r = -0.429). In addition, diatoms were also positively correlated with the abundance of the tintinnids (r = 0.443) (Table 3).
DISCUSSION
This study showed a temporal variability in most of the data obtained, both physically and chemically as well as biologically. From the abiotic perspective, results reflected a clear trend related to the climatic variability that characterizes Costa Rica: on one hand, a dry season marked by increased temperatures and the influence of the wind mixing the waters, which generates greater turbulence in the water column and resuspension of sediments, and, on the other hand, a rainy season characterized by a high input of fresh water and sediment into the sea (Lizano & Vargas, 1994; Brenes et al. 2001). According to Voorhis et al. (1983) and Brenes (2001), the influence of the discharge of rivers such as the Tempisque, Barranca, and Grande de Tárcoles is a determining factor in the Gulf of Nicoya, since it increases the variability of physical and chemical factors of the water, due to the constant input of fresh water in the estuary, which fluctuates depending on the weather period.
The variation in the percentage of oxygen saturation also reflected constant changes experienced by the estuary of the Gulf of Nicoya throughout an annual cycle. The values obtained from the percentages of oxygen saturation during the rainy season agree with those obtained by Kress et al. (2001) and Epifanio et al. (1983), who determined that the Gulf of Nicoya is an unsaturated estuary when there is an increase in rainfall in the area, caused by the consumption of oxygen by the organic matter that enters through the runoff.
The Secchi disk depth was closely related to the behavior of the phytoplankton community, presenting the highest values when the microalgae concentration was lower, and vice versa. This is confirmed with the negative correlation between the concentration of diatoms and the Secchi disk depth. In addition, according to Medina (1995), Litchman & Klausmeier (2008) and Aktan (2011), the extinction of light in water is proportional to the abundance of phytoplankton. This condition is reflected only in the diatoms since this is the dominant group as far as concentration, unlike the other phytoplankton organisms.
The richness of the phytoplankton in the Paquera-Tambor MARF reflects an important group of organisms, mainly belonging to diatoms and dinoflagellates. This research, as well as others in other coastal areas (Eker & Kideyş, 2000; Peña & Pinilla, 2002; Varela & Prego, 2003; Álvarez-Góngora & Herrera-Silveira, 2006; Simon et al. 2009; Haraguchi et al. 2015) reported higher concentrations of diatoms with respect to the other phytoplankton groups identified, with the dinoflagellates being the next highest group in terms of the number of present species. This has already been noted for other coastal estuarine environments and is also evidenced by the fact that the most common species in this study belonged to these phytoplankton groups. Rochelle-Newall et al. (2011) evaluated the distribution and diversity of phytoplankton in northern Viet Nam estuarine environments and found that between 43% and 99% of the samples were diatoms. In addition, Cabrita (2014) determined the richness of the phytoplankton community as an indicator of changes related to the drainage in the Tagus estuary in Portugal, determining that groups such as the diatoms, dinoflagellates, and cryptophytes represent a total of 92-99% of the phytoplankton community.
On the other hand, several authors have determined that the dominant numbers of diatoms species is strongly related to increased concentrations of nutrients (Ishizaka et al. 1986; Day et al. 2012), which could be associated to the characteristics of the Gulf of Nicoya, where the dynamics of the estuary with constant inputs of freshwater, mangrove ecosystems near shore and the processes of mixing waters (Brenes & León, 1995) favor a constant flow of nutrients (Magnone et al. 2015; Sin et al. 2015).
The low numbers of prasinophytes, cyanophytes and chromophytes can be related to the fact that they are phytoplankton groups common in coastal ecosystems of oligotrophic nature. According to several authors, these phytoplanton groups represent some of the most important nanoplankton groups in the oligotrophic systems (Puigserver et al. 2002; Resende et al. 2007; Aktan, 2011; Huete-Ortega et al. 2010; Schlüter et al. 2011).
The presence of tintinnids in the waters of the Paquera-Tambor MARF is related to the adaptation of this group of organisms to areas where freshwater and saltwater converge (Zhang et al. 2015). However, few studies of tintinnids have been reported in tropical areas, as subtropical to temperate areas are considered to be the comfort zone of this group of ciliates (Quevedo et al. 2003; Safi et al. 2007; Santoferrara & Alder, 2009; Li et al. 2015).
The positive relationship between diatoms and tintinnids is due to a primary trophic relationship, since it was observed that the increase in the concentration of diatom cells coincides with the increase in the abundance of ciliates, suggesting a relevant grazing process in the Paquera-Tambor MARF (Stelfox-Widdicombe et al. 2004). This is consistent with what was observed by Widdicombe et al. (2010), who determined that the seasonal pattern of abundance of the ciliates in the Western English Channel was similar to the population dynamics of the diatoms and the phytoflagellates.
The presence of algal bloom forming species in the waters of the Paquera-Tambor MARF proves the wide distribution of these organisms in the Gulf of Nicoya, where the presence of dinoflagellates Cochlodinium catenatum, Pyrodinium bahamense var. compressum, Alexandrium monilatum, and diatoms Pseudo-nitzschia spp. has been reported from the inside to the outside area of the Gulf of Nicoya (García, 2005; Calvo et al. 2014). In the case of dinoflagellates, these species are common in the waters of the Gulf of Nicoya; C. catenatum is a recurring species in the production of red tides in the Gulf (García, 2005) as well as P. bahamense var. compressum (Víquez & Hargraves, 1995). In the case of A. monilatum, it was not until 2005 that the first red tide caused by this species was reported for this area (Calvo et al. 2005).
In general, results reflect that the Paquera-Tambor MARF is an important ecological area, since the presence of different microalgae groups and zooplankton organisms (tintinnids) are the basis of the food chain in the area (Magnone et al. 2015; Feng et al. 2015; Rakshit et al. 2016), and, therefore, favor the following levels of the food chain. In addition, given that this is an important area for aquaculture and fishery production, this study is of vital importance to ensure an ecosystem assessment that allows for the recognition of guidelines for a comprehensive management of the marine area for responsible fishing.
This type of monitoring should be maintained in Marine Areas for Responsible Fishing, since they are areas susceptible to harmful and toxic algal blooms, and, consequently, can affect the economy of the communities exploiting them.
ACKNOWLEDGMENTS
We would like to thank the fishermen and the managers of the Marine Area for Responsible Fishing for their willingness to conduct the study as a complement to their management plan and the reviewers for their valuable suggestions.
BIBLIOGRAPHY
Aktan, Y. (2011). Large-scale patterns in summer surface water phytoplankton (except picophytoplankton) in the Eastern Mediterranean. Estuar. Coast. Shelf Sci., 91, 551-558. http://dx.doi.org/10.1016/j.ecss.2010.12.010
Álvarez-Góngora, C. & Herrera-Silveira, J. A. (2006). Variations of phytoplankton community structure related to water quality trends in a tropical karstic coastal zone. Mar. Pollut. Bull., 52, 48-60. http://dx.doi.org/10.1016/j.marpolbul.2005.08.006
Araya, H., Vásquez, A., Marín, B., Palacios, J. A., Soto-Rojas, R., Mejía-Arana, F., Shimazu, Y. & Hiramatsu, K. (2007). Reporte no.1/2007 del Comité de Evaluación de Poblaciones: Reporte del Manejo de los Recursos Pesqueros en el Golfo de Nicoya. Puntarenas, Costa Rica: UNA-JICA-INCOPESCA.
Brenes, C. (2001). Fundamentos de Oceanografía Descriptiva: Aplicación al Istmo Centroamericano. Bluefields, Nicaragua: Proyecto para el Desarrollo Integral de la Pesca Artesanal en la Región Autónoma Atlántico Sur (DIPAL).
Brenes, C. & León, S. (1995). Hidrografía del Golfo de Nicoya. In J. Zarnorro (Ed.), Actas del Simposium Ecosistema de Manglares en el Pacífico Centroamericano y sus Recursos de Post-larvas de Camarones Peneidos (pp. 39-47). San Salvador, El Salvador: PRADEPESCA.
Brenes, C. L., León, S. & Chávez, J. (2001). Variación de las propiedades termohalinas en el Golfo de Nicoya, Costa Rica. Rev. Biol. Trop., 49, 145-152.
Cabrita, M. T. (2014). Phytoplankton community indicators of changes associated with dredging in the Tagus estuary (Portugal). Environ. Pollut., 191, 17-24. http://dx.doi.org/10.1016/j.envpol.2014.04.001
Calvo, E., Víquez, R. & García, A. (2005). Alexandrium monilatum (Howell) Balech bloom in the Gulf of Nicoya, Puntarenas. Harmful Algae News. IOC-UNESCO, 29, 1-2.
Calvo, E., Boza, J. & Berrocal, K. (2014). Efectos de El Niño y La Niña sobre el comportamiento del microfitoplancton marino y las variables fisicoquímicas durante el 2008 a 2010 en el Golfo de Nicoya, Costa Rica. Rev. Cienc. Mar. Cost., 6, 115-133. http://dx.doi.org/10.15359/revmar.8-1.9
Chrétiennot-Dinet, M. J. (1990). Chlorarachniophycées, Chlorophycées, Chrysophycées, Cryptophycées, Euglénophycées, Eustigmatophycées, Prasinophycées, Prymnésiophycées, Rhodophycées et Tribophycées. In A. Sournia (Ed.), Atlas Du Phytoplancton Marin. Volume II (pp. 1-261). Paris, France: Éditions du Centre National de la Recherche Scientifique.
Cupp, E. (1937). Marine Plankton Diatoms of the West Coast of North America. California, USA: University of California Press.
Dawes, C. (1991). Botánica Marina. Distrito Federal, México: Editorial Limusa, S.A.
Day, J. G., Slocombe, S. P. & Stanley, M. S. (2012). Overcoming biological constraints to enable the exploitation of microalgae for biofuels. Bioresource Technol., 109, 245-251. http://dx.doi.org/10.1016/j.biortech.2011.05.033
Domingues, R., Barbosa, A. & Galvão, H. (2008). Constraints on the use of phytoplankton as a biological quality element within the Water Framework Directive in Portuguese waters. Mar. Pollut. Bull., 56, 1389-1395. http://dx.doi.org/10.1016/j.marpolbul.2008.05.006
Eker, E. & Kideyş, A. E. (2000). Weekly variations in phytoplankton structure of a Harbour in Mersin Bay (north-eastern Mediterranean). Turk. J. Bot., 24, 13-24.
Epifanio, C. E., Maurer, D. & Dittel, A. I. (1983). Seasonal changes in nutrients and dissolved oxygen in the Gulf of Nicoya, a tropical estuary on the Pacific coast of Central America. Hydrobiologia, 101, 231-238. http://dx.doi.org/10.1007/BF00009879
Feng, M., Zhang, W., Wang, W., Zhang, G., Xiao, T. & Xu, H. (2015). Can tintinnids be used for discriminating water quality status in marine ecosystems? Mar. Pollut. Bull., 101(2), 549-555. http://dx.doi.org/10.1016/j.marpolbul.2015.10.059
García, A. (2005). Biomasa fitoplanctónica durante un florecimiento algal en el Golfo de Nicoya, Costa Rica. Unpublished Licentiate Thesis, Universidad Nacional, Costa Rica.
Hansen, B., Eliasen, S. K., Gaard, E. & Larsen, K. M. H. (2005). Climatic effects on plankton and productivity on the Faroe Shelf. ICES J. Mar. Sci., 62, 1224-1232. http://dx.doi.org/10.1016/j.icesjms.2005.04.014
Haraguchi, L., Carstensen, J., Abreu, P. C. & Odebrecht, C. (2015). Long-term changes of the phytoplankton community and biomass in the subtropical shallow Patos Lagoon Estuary, Brazil. Estuar. Coast. Shelf Sci., 162, 76-87. http://dx.doi.org/10.1016/j.ecss.2015.03.007
Hoff, N. T., Figueira, R. C. L. & Abessa, D. M. S. (2015). Levels of metals, arsenic and phosphorus in sediments from two sectors of a Brazilian Marine Protected Area (Tupinambás Ecological Station). Mar. Pollut. Bull., 91, 403-409. http://dx.doi.org/10.1016/j.marpolbul.2014.10.044
Hoppenrath, M., Elbrächter, M. & Drebes, G. (2009). Marine phytoplankton: Selected microphytoplankton species from the North Sea around Helgoland and Sylt. Stuttgart, Germany: Kleine Senckenberg-Reihe 49.
Horner, R. (2002). A Taxonomic Guide to Some Common Marine Phytoplankton. Briston, England: Biopress Ltd.
Hu, H., Zhang, J. & Chen, W. (2011). Competition of bloom-forming marine phytoplankton at low nutrient concentrations. J. Environ. Sci., 23, 656-663. http://dx.doi.org/10.1016/S1001-0742(10)60459-7
Huete-Ortega, M., Marañón, E., Varela, M. & Bode, A. (2010). General patterns in the size scaling of phytoplankton abundance in coastal waters during a 10-year time series. J. Plankton Res., 32, 1-14. http://dx.doi.org/10.1093/plankt/fbp104
Ishizaka, J., Takahashi, M. & Ishimura, S. (1986). Changes in the growth rate of phytoplankton in local upwelling around the Izu Peninsula, Japan. J. Plankton Res., 8(l), 169-181. http://dx.doi.org/10.1093/plankt/8.1.169
Jeffrey, S. W., Montoura, R. F. C. & Wright, S. W. (1997). Phytoplankton pigments in oceanography guidelines to modern methods. Paris, France: UNESCO.
Knox, G. (2000). The ecology of seashores. Florida, USA: CRC Press. http://dx.doi.org/10.1201/9781420042634
Kress, N., León, S., Brenes, C., Brenner, S. & Arroyo, G. (2001). Horizontal transport and seasonal distribution of nutrients, dissolved oxygen and chlorophyll-a in the Gulf of Nicoya, Costa Rica: a tropical estuary. Cont. Shelf Res., 0, 1-16.
Li, Y., Chen, Y., Song, B., Olson, D., Yu, N. & Chen, L. (2009). Ecosystem structure and functioning of Lake Taihu (China) and the impacts of fishing. Fish. Res., 95, 309-324. http://dx.doi.org/10.1016/j.fishres.2008.09.039
Li, H., Zhao, Y., Chen, X., Zhang, W., Xu, J., Li, J. & Xiao, T. (2015). Interaction between neritic and warm water tintinnids in surface waters of East China Sea. Deep-Sea Research II, 124, 84-92. http://dx.doi.org/10.1016/j.dsr2.2015.06.008
Litchman, E. & Klausmeier, C. A. (2008). Trait-Based Community Ecology of Phytoplankton. Annu. Rev. Ecol. Evol. Syst., 39, 615-639. http://dx.doi.org/10.1146/annurev.ecolsys.39.110707.173549
Lizano, O. & Vargas, J. (1994). Distribución espacio-temporal de la salinidad y la temperatura en la parte interna del Golfo de Nicoya. Tecnol. Mar., 12(2), 3-16.
MacIntery, H. L., Kana, T. M. & Geider, R. J. (2000). The effect of water motion on short-term rates of photosynthesis by marine phytoplankton. Trends Plant. Sci. Rev., 5, 12-17. http://dx.doi.org/10.1016/S1360-1385(99)01504-6
MAG. Ministerio de Agricultura y Ganadería. (2009). Reglamento para el Establecimiento de las Áreas Marinas de Pesca Responsable y Declaratoria de Interés Público Nacional de las Áreas Marinas de Pesca Responsable. Decreto Nº 35502-MAG. (Published in the Gazette N° 191, October 01). San José, Costa Rica: Imprenta Nacional.
Magnone, L., Bessonart, M., Gadea, J. & Salhi, M. (2015). Trophic relationships in an estuarine environment: A quantitative fatty acid analysis signature approach. Estuar. Coast. Shelf Sci., 166, 24-33. http://dx.doi.org/10.1016/j.ecss.2014.12.033
Mann, K. H. (2000). Ecology of Coastal Waters: Implications for Management. Massachusetts, USA: Blackwell Science, Inc.
Medina, L. (1995). Análisis multidisciplinar del ecosistema costero insular, balance energético, capa de mezcla y modelo biológico. Unpublished Doctoral Dissertation, Universidad de Las Palmas de Gran Canaria, Spain.
Nybakken, J. W. (2001). Marine Ecology: An ecological approach. New York, USA: Benjamin Cummings, Addison Wesley Longman.
OCEANA. (2013). Áreas marinas protegidas: una herramienta para combatir la sobrepesca y conservar los ecosistemas marinos. Oceana, 4, 1-15.
Ojeda, A. (2006). Dinoflagelados de Canarias: Estudio Taxonómico y Ecológico. Tenerife, Islas Canarias, Spain: Litografía Romero, S.L.
Peña, V. & Pinilla, G. A. (2002). Composición, distribución y abundancia de la comunidad fitoplanctónica de la ensenada de Utría, Pacífico colombiano. Rev. Biol. Mar. Oceano., 37, 67-81.
Puigserver, M., Ramon, G., Moya, G. & Martínez-Taberner, A. (2002). Planktonic chlorophyll a and eutrophication in two Mediterranean littoral systems (Mallorca Island, Spain). Hydrobiologia, 475/476, 493-504. http://dx.doi.org/10.1023/A:1020368215511
Quevedo, M., Viesca, L., Anadon, R. & Fernández, E. (2003). The protistan microzooplankton community in the oligotrophic north-eastern Atlantic: large- and mesoscale patterns. J. Plankton Res., 25, 551-563. http://dx.doi.org/10.1093/plankt/25.5.551
Rakshit, D., Ganeshb, P. S. & Sarkar, S. K. (2016). Choreotrich ciliate tintinnid (Protozoa: Ciliophora) in a tropical meso-macrotidal estuary, eastern part of India. Reg. Stud. Mar. Sci., 3, 89-100. http://dx.doi.org/10.1016/j.rsma.2015.06.003
Resende, P., Miranda, U., Gonçalves, F. & Pereira, M. J. (2007). Distribution and ecological preferences of diatoms and dinoflagellates in the west Iberian Coastal zone (North Portugal). Acta Oecol., 32, 224-235. http://dx.doi.org/10.1016/j.actao.2007.05.004
Ricard, M. (1987). Diatomophycées. In A. Sournia (Ed.), Atlas du Phytoplancton Marin. Volume II (pp. 262-296). Paris, France: Éditions du Centre National de la Recherche Scientifique.
Rochelle-Newall, E. J., Chu, T. V., Pringault, O., Amouroux, D., Arfi, R., Bettarel, Y. T., Bouvier, C., Got, P., Nguyen, T. M. H., Mari, X., Navarro, P., Duong, T. N., Cao, T. T. T., Pham, T. T., Ouillon, S. & Torréton, J. P. (2011). Phytoplankton distribution and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Mar. Pollut. Bull., 62, 2317-2329. http://dx.doi.org/10.1016/j.marpolbul.2011.08.044
Round, F. E., Crawford, R. M. & Mann, D. G. (1990). The Diatoms: Biology & Morphology of the Genera. New York, USA: Cambridge University Press.
Safi, K., Griffiths, F. & Hall, J. A. (2007). Microzooplankton composition, biomass and grazing rates along the WOCE SR3 line between Tasmania and Antarctica. Deep-Sea Res. Pt. I, 54, 1025-1041. http://dx.doi.org/10.1016/j.dsr.2007.05.003
Santoferrara, L. & Alder, V. (2009). Abundance trends and ecology of planktonic ciliates of the south-western Atlantic (35-63 o S): a comparison between neritic and oceanic environments. J. Plankton Res., 31, 837-851. http://dx.doi.org/10.1093/plankt/fbp033
Schlüter, L., Henriksen, P., Nielsen, T. G. & Jakobsen, H. H. (2011). Phytoplankton composition and biomass across the southern Indian Ocean. Deep-Sea Res. Pt. I, 58, 546-556. http://dx.doi.org/10.1016/j.dsr.2011.02.007
Simon, N., Cras, A. L., Foulon, E. & Lemée, R. (2009). Diversity and evolution of marine phytoplankton. C.R. Biol., 332, 159-170. http://dx.doi.org/10.1016/j.crvi.2008.09.009
Sin, Y., Lee, E., Lee, Y. & Shin, K. H. (2015). The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci., 163, 279-289. http://dx.doi.org/10.1016/j.ecss.2014.12.028
Sournia, A. (1986). Atlas du Phytoplancton Marin. Volume I: Introduction, Cyanophycées, Dictyochophycées, Dinophycées et Raphidophycées. Paris, France: Éditions du Centre National de la Recherche Scientifique.
Spatharis, S. & Tsirtsis, G. (2010). Ecological quality scales based on phytoplankton for implementation of Water Framework Directive in the Eastern Mediterranean. Ecol. Indic., 10, 840-847. http://dx.doi.org/10.1016/j.ecolind.2010.01.005
SPSS. (2008). SPSS Statistics for Windows, Version 17.0. Chicago, USA: SPSS Inc.
Steingrund, P. & Gaard, E. (2005). Relationship between phytoplankton production and cod production on the Faroe Shelf. ICES J. Mar. Sci., 62(2), 163-176. http://dx.doi.org/10.1016/j.icesjms.2004.08.019
Stelfox-Widdicombe, C. E., Archer, S. D., Burkill, P. H. & Stefels, J. (2004). Microzooplankton grazing in Phaeocystis and diatom-dominated waters in the southern North Sea in spring. J. Sea Res., 51, 37-51. http://dx.doi.org/10.1016/j.seares.2003.04.004
Throndsen, J., Hasle, G. R. & Tangen, K. (2007). Phytoplankton of Norwegian Coastal Waters. Oslo, Norway: Almater Forlag As.
Tomas, C. (1997). Identifying Marine Phytoplankton. San Diego, USA: Academic Press.
Valiela, I. (1995). Marine ecological process. New York, USA: Springer. http://dx.doi.org/10.1007/978-1-4757-4125-4
Varela, M. & Prego, R. (2003). Hydrography and phytoplankton in an isolated and non-pristine ria area: the A Coruña Harbour (NW Spain). Acta Oecol., 24, 113-124. http://dx.doi.org/10.1016/S1146-609X(03)00048-1
Víquez, R. & Hargraves, P. (1995). Annual cycle of potentially harmful dinoflagellates in the Golfo de Nicoya, Costa Rica. Bull. Mar. Sci., 57(2), 467-475.
Voorhis, A., Epifanio, C., Maurer, D., Dittel, A. & Vargas, J. (1983). The estuarine character of the Gulf of Nicoya, an embayment on the Pacific coast of Central America. Hydrobiologia, 99, 225-237. http://dx.doi.org/10.1007/BF00008774
Widdicombe, C. E., Eloire, D., Harbour, D., Harris, R. P. & Somerfield, P. J. (2010). Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res., 32, 643-655. http://dx.doi.org/10.1093/plankt/fbp127
Zhang, C., Zhang, W., Ni, X., Zhao, Y., Huang, L. & Xiao, T. (2015). Influence of different water masses on planktonic ciliate distribution on the East China Sea shelf. J. Mar. Syst., 141, 98-111. http://dx.doi.org/10.1016/j.jmarsys.2014.09.003

Revista Ciencias Marinas y Costeras está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.