Rev. Mar. Cost. ISSN 1659-455X. Vol. 9 (1): 9-21, Enero-Junio 2017.
DOI: http://dx.doi.org/10.15359/revmar.9-1.1
Spontaneous spawning in captivity of the first generation hatchery-produced weakfish, Cynoscion squamipinnis (Perciformes: Sciaenidae)
Desove espontáneo en cautiverio de la primera generación criada en laboratorio de corvina aguada, Cynoscion squamipinnis (Perciformes: Sciaenidae)
Jorge Boza Abarca1*, Marvin Ramírez Alvarado1, Emilia Calvo Vargas1 and Karen Berrocal Artavia1
1 Juan Bertoglia Richards Marine Biology Station, School of Biological Sciences, Universidad Nacional, Puntarenas, Costa Rica. jorge.boza.abarca@una.cr* and jboza07@gmail.com
Recibido: 23 de junio de 2016
Corregido: 16 de setiembre de 2016
Aceptado: 19 de octubre de 2016
ABSTRACT
The aim of this study was to evaluate the spontaneous spawning of the first generation of weakfish (Cynoscion squamipinnis) reared in captivity in order to conduct restocking programs and mariculture projects. Juveniles obtained from spontaneous spawning of wild fish caught in the Gulf of Nicoya were reared at the Laboratory of Marine Fish Reproduction and Culture in the Juan Bertoglia Richards Marine Biology Station, Universidad Nacional, Puntarenas, Costa Rica. Fish were kept in 18 t tanks, with constant water exchange (100% per day), at a temperature of 26.73 ± 1.15°C, 32.60 ± 2.69 PSU salinity, and 6.20 ± 0.61 mg/L dissolved oxygen. Every two months croakers were anesthetized and examined for sexual maturity. In January 2011, maturity of one female was confirmed by ovarian biopsies, and oocytes of 0.4-0.6 mm diameter were observed; also fluid semen was obtained from males through abdominal massage. In March 2011, a period of spontaneous spawning began, without any environmental or hormonal stimulation. In total 34 spawns were recorded from March to October 2011, for an average of 85,996 eggs, with a maximum production of 461,000 eggs and a minimum of 4,500 eggs. Fertilization percentage was 10-80%, with an average of 53.09 ± 26.97%. No significant correlations were found between spawns and environmental variables such as temperature, salinity, dissolved oxygen, tides, moon phases and precipitation.
Keywords: Reproduction, mariculture, fertilization, sexual maturity, environmental variables.
Resumen
El objetivo de este estudio fue evaluar los desoves espontáneos de la primera generación de corvina aguada (Cynoscion squamipinnis) criada en cautiverio con el fin de realizar programas de repoblamiento y proyectos de maricultura. Los juveniles obtenidos de desoves espontáneos de peces silvestres capturados en el Golfo de Nicoya fueron criados en el Laboratorio de Reproducción y Cultivo de Peces Marinos de la Estación de Biología Marina, Universidad Nacional, Puntarenas, Costa Rica. Los peces se mantuvieron en tanques de 18 t, con recambio de agua (100% diario), temperatura de 26.73 ± 1.15°C, salinidad de 32.60 ± 2.69 UPS y oxígeno disuelto de 6.20 ± 0.61 mg/L. Cada dos meses las corvinas fueron anestesiadas y examinadas para determinar su madurez sexual. En enero del año 2011, se comprobó mediante biopsias ováricas que una de las hembras estaba madura y se observaron ovocitos de 0.4-0.6 mm de diámetro, y mediante masaje abdominal se obtuvo semen fluido de los machos. En marzo del año 2011 comenzó un periodo de desoves espontáneos, sin realizar ningún estímulo ambiental u hormonal. En total se registraron 34 desoves de marzo a octubre del año 2011, obteniéndose un promedio de 85 996 huevos, con un máximo de 461 000 huevos y un mínimo de 4 500 huevos. El porcentaje de fertilización fue de 10-80%, con un promedio de 53.09 ± 26.97%. No se encontraron correlaciones significativas entre los desoves y las variables ambientales de la temperatura, la salinidad, el oxígeno disuelto, las mareas, las fases lunares y la precipitación.
Palabras claves: Reproducción, maricultura, fertilización, madurez sexual, variables ambientales.
INTRODUCTION
Weakfish, Cynoscion squamipinnis (Sciaenidae), is one of the 34 species found along the pacific coast of the Americas, and, in Costa Rica, is one of the most captured species in the Gulf of Nicoya (Chacón et al. 2007; Boza-Abarca et al. 2016a; b). The amount captured with a trammel was 166 MT in 2002, reaching a maximum of 258 MT in 2003 but decreasing to 142 MT in 2005 (Chacón et al. 2007).
Most croaker species mature in captivity, but fail to ovulate or spawn. This phenomenon is called reproductive dysfunction and occurs due to the lack of the luteinizing hormone (LH) released at the end of the vitellogenesis (Mylonas et al. 2013a; b). In order to solve this problem, hormones that trigger the release of LH have been used, consequently stimulating the release of sex steroids. Hormonal treatments allow the conclusion of the process through ovulation and spawning (Cárdenas, 2011; 2012; Papadakis et al. 2013). In the Sciaenidae family, Tucker (1998) reported 12 species induced with treatments such as Human Chorionic Gonadotropin (hCG), Suspension of Pituitary (SP), and Gonadotropin-Releasing Hormone Antagonist (GnRHa), particularly species like Atractoscion nobilis, Cynoscion nebulosus, C. regalis, and Sciaenops ocellatus, all of which were of commercial interest or were reproduced in captivity because they had been overfished and had been included in restocking programs.
Argyrosomus regius has been selected as a potential species for the development of mariculture in the Mediterranean (Mylonas et al. 2013a; b); however, it presents the problem of failing to spawn in captivity (Schiavone et al. 2012). This species has been successfully induced with GnRHa implants (Duncan et al. 2012; Mylonas et al. 2013b), using Luteinizing Hormone-Releasing Hormone Analog (LHRHa) injections (Gamzis & Neke, 2008; Ballagh et al. 2011; Cárdenas, 2012; Duncan et al. 2012; 2013; Mylonas et al. 2013a), hCG injections (García-Alonso & Vizziano, 2004), or GnRHa or GnRH injections and implants (Jiménez et al. 2005; Duncan et al. 2007a; b; Duncan et al. 2008; Cárdenas et al. 2008; Cárdenas, 2011; 2012; Duncan et al. 2012). Other Sciaenidae species have been induced to spawning; for instance, Umbrina cirrosa has been induced with GnRHa (Jiménez et al. 2005; Koumoundouros et al. 2005), Argyrosomus japonicus with LHRHa (Jiménez et al. 2005; Musson & Kaiser, 2014), and Micropogonias furnieri with hCG (García-Alonso & Vizziano, 2004). In Costa Rica, the spotted rose snapper, Lutjanus guttatus, was induced to spawning using carp pituitary extract and hCG in different doses (Valverde & Boza, 1999; Boza-Abarca et al. 2008; 2011); however, after induction, which produced only one spawning, fish maintained in captivity produced serial spawns for three months (Boza-Abarca et al. 2008).
Other croakers spawn voluntarily in captivity: Atractoscion nobilis (California), Cynoscion nebulosus (Texas and Florida), Cynoscion xanthulus (Texas), Equetus acuminatus (Florida), Equetus lanceolatus (Florida), Pogonias cromis (Texas), Pseudosciaena polyactis (Korea), Sciaenops ocellatus (Florida, Texas, Taiwan) (Thomas et al. 1995; Tucker, 1998; Jiménez et al. 2005; López & Durazo, 2008), and Rachycentron canadum (Bennetti et al. 2007).
In Costa Rica, captured weakfish, C. squamipinnis, spawned spontaneously without any hormone treatment (Boza et al. 2016a), and juveniles produced were grown out (Boza-Abarca et al. 2016b) and maintained in captivity as the first hatchery-produced generation.
The objective of this study was to analyze the spontaneous spawning of the first generation of hatchery-produced weakfish, C. squamipinnis, in order to evaluate the reproductive potential in captivity of the species for future restocking and mariculture projects in the Gulf of Nicoya.
MATERIALS AND METHODS
In April 2010, juvenile weakfish (C. squamipinnis) obtained as a result of spontaneous spawning in captivity of fish caught in the Gulf of Nicoya were placed in a broodstock tank at the Juan Bertoglia Richards Marine Biology Station, at Universidad Nacional, Costa Rica. Fish at an initial average weight of 132.6 ± 32.96 g (n = 12, average ± SD), maximum weight of 219.30 g and minimum weight of 78.50 g, as well as a total length of 23.34 ± 1.65 cm, maximum length of 27.00 cm and minimum length of 20.50 cm, were maintained at a density of 0.66 fish/m3 (88.39 g/m3) in an 18 t external cylindrical fiberglass tank. Specimens adapted to spawning conditions (Saran Wrap cover with 80% shade), constant aeration (20 psi), egg collector with fine mesh (250-500 µm), and daily water exchange (80%). Fish were fed chopped sardines daily (2% body weight). In the broodstock tank, salinity (PSU) was measured daily with an SR5-E refractometer, while temperature (°C) and dissolved oxygen (mg/L) were measured with a YSI DO200 multiparameter. The egg collector consisted of a fiberglass container covered with a fine mesh of less than 500 µm (20 L volume) and placed in the tank outlet.
Maturity was observed every two months between April and October 2010, in order to reduce fish handling, and then in January 2011. Fish were not fed the day before sampling and were anesthetized in a tank with methanesulfonate (MSS) (0.1 g L-1) or with clove oil (0.1 ml L-1). In order to evaluate oocyte development, ovarian biopsies were taken by inserting a plastic cannula in the urogenital pore and applying suction (n = 4 females). Ovarian biopsies were examined using a stereoscope (10X) to determine stage of maturity and measure oocyte diameter (n = 30). Male maturation (n = 8) was determined by the release of semen through abdominal massage (Boza-Abarca et al. 2008; 2011; Boza-Abarca et al. 2016a).
The time when spawning occurred was recorded based on the presence of eggs on the surface of the tank or the collector. Each morning eggs were found in the collector and were later placed mixed in a 2 L cylindrical container; in addition, 3 subsamples of 1 mL subsamples were taken to determine the number of fertile and infertile eggs. Fertile eggs were separated from infertile eggs based on buoyancy, and were placed in a 1,000 L tank for later larval sampling.
Date, temperature, salinity, and dissolved oxygen were determined for each spawning observed. These values were supplemented with annual data of high and low tide fluctuation (m), moon phases, precipitation (mm), and total number of eggs, fertilization percentage and the number of juveniles produced per spawning. In order to determine relationships between one or more variables, a Spearman’s multiple correlation analysis was conducted using SPSS, version 17.0 (SPSS, 2006). In addition, daily values of temperature, salinity, dissolved oxygen, high and low tide, and precipitation during the study period were used in order to relate them with the spawns and to observe trends.
RESULTS
Broodstock reared in the laboratory seeded in February 2010 reached an average weight of 339.44 ± 79.98 g, with a maximum of 432.70 g (total length 36.00 cm) and a minimum of 189.50 g (total length 29.00 cm), in October 2010. When the ovarian sample was taken, oocyte diameter was measured ranging between 0.4 and 0.6 mm in one of the marked females (4 females). However, the ovarian samples extracted from the other females did not show oocytes in the advanced stages of vitellogenesis (Boza-Abarca et al. 2016a). On February 8, 2011, the first spontaneous spawning occurred, obtaining 28,500 eggs, and the process continued during the following months with a total of 34 reported spawns until October 2011 (Table 1, Fig. 1). Fish were not sampled during the spontaneous spawning period to avoid causing them stress that would have interrupted their spawning; therefore, it was not possible to determine how many females participated in the spontaneous spawning. Nonetheless, taking into account that during the previous evaluation in October 2010 advanced oocytes (vitellogenic) were observed in only one female, it is assumed that this female was the one that spawned. Regarding males (8 males), 40% released semen after abdominal massage (n = 3); however, it was not fluid.
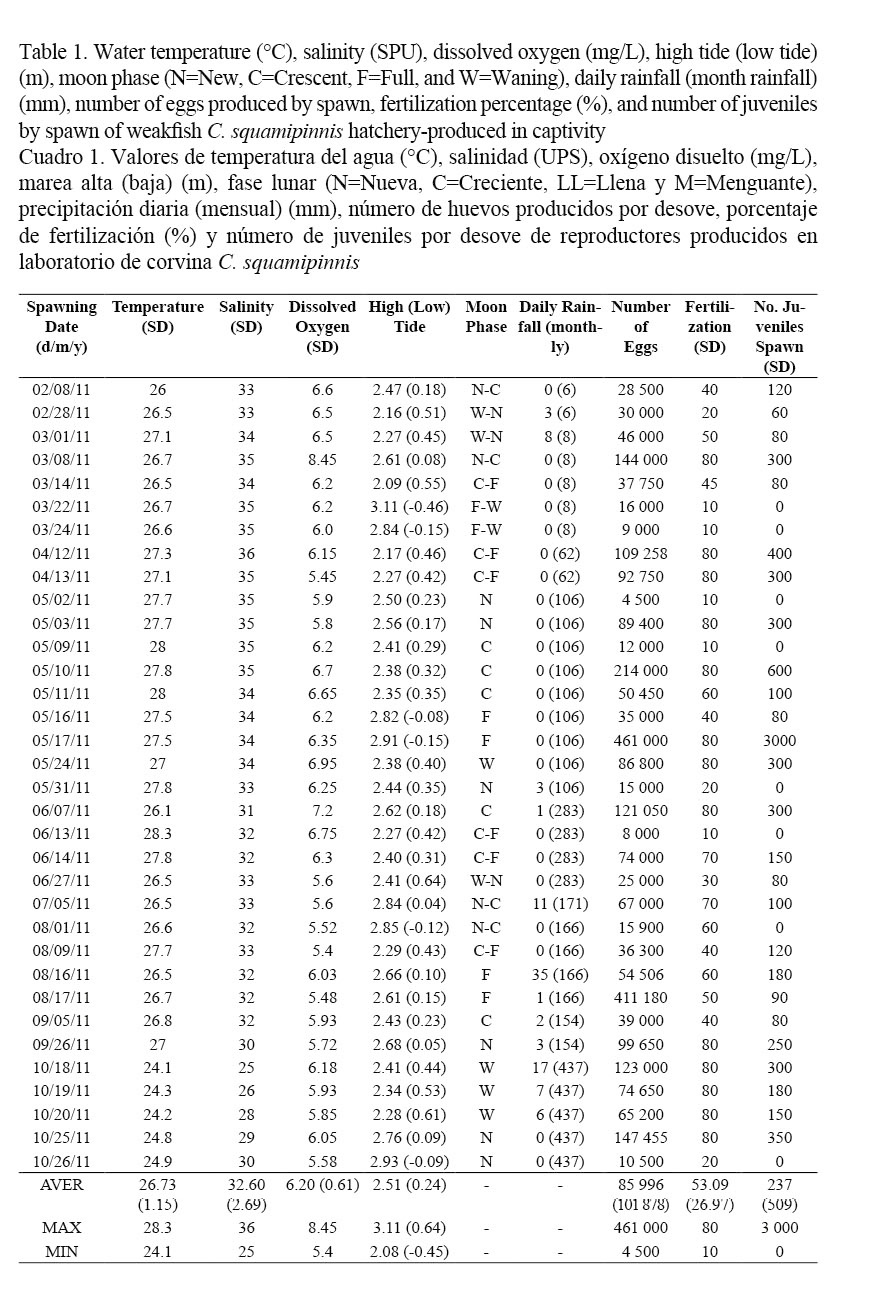
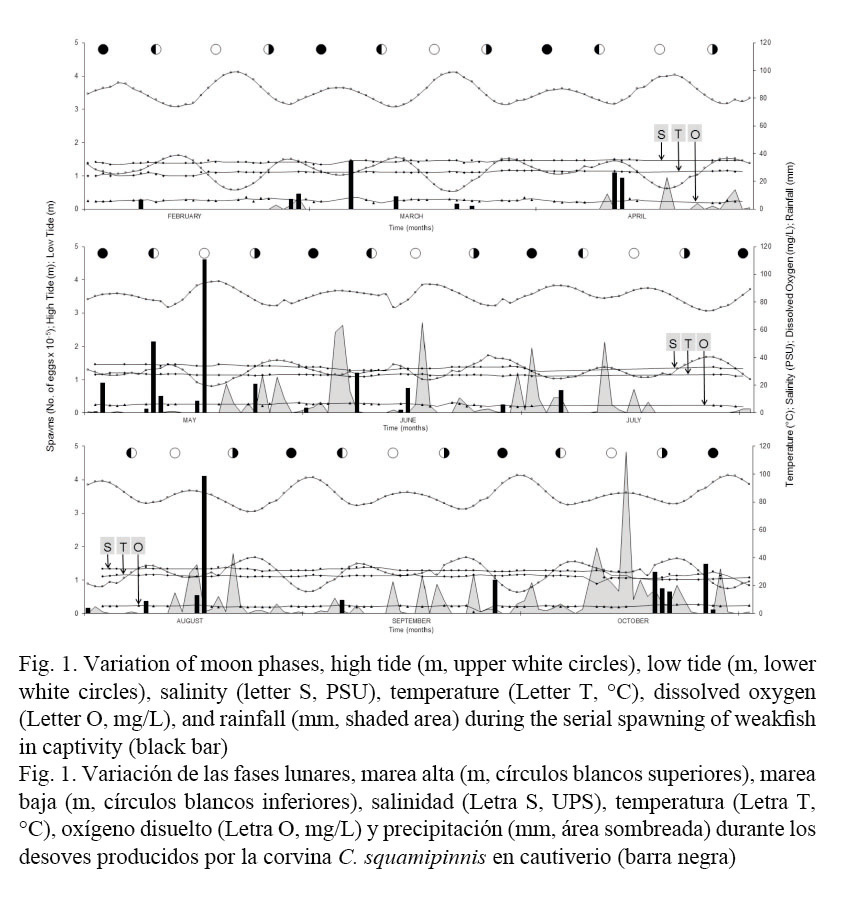
Spawns occurred continuously up to three times in different days, the first one being a premonitory instance smaller than the following events (Fig. 1). Spawns occurred at an average temperature of 26.73 ± 1.15°C, with a maximum of 28.30°C and a minimum of 24.1°C, average salinity of 32.60 ± 2.69 PSU, with a maximum of 36 PSU and a minimum of 25 PSU; average dissolved oxygen of 6.20 ± 0.61 mg L-1, with a maximum of 8.45 mg L-1 and a minimum of 5.4 mg L-1. Average high tides and low tides were 3.11 ± 0.64 m and 2.08 ± 0.45 m, respectively.
The majority of spawns were related to the crescent and full moon phases (18 spawns, Table 1, Fig. 1), followed by the new moon phase (13 spawns). Most of the spawns took place in days with no precipitation (0 mm, 22 spawns). An average of 85,996 eggs was obtained, having a maximum of 461,000 eggs laid with a fertilization rate of 80%, and a minimum of 4,500 eggs with a rate of 10% fertilization. Average fertilization rate was 53.09 ± 26.97%. A total of 8,050 juveniles were produced; in some of the spawns fertilization did occur but no juveniles were obtained.
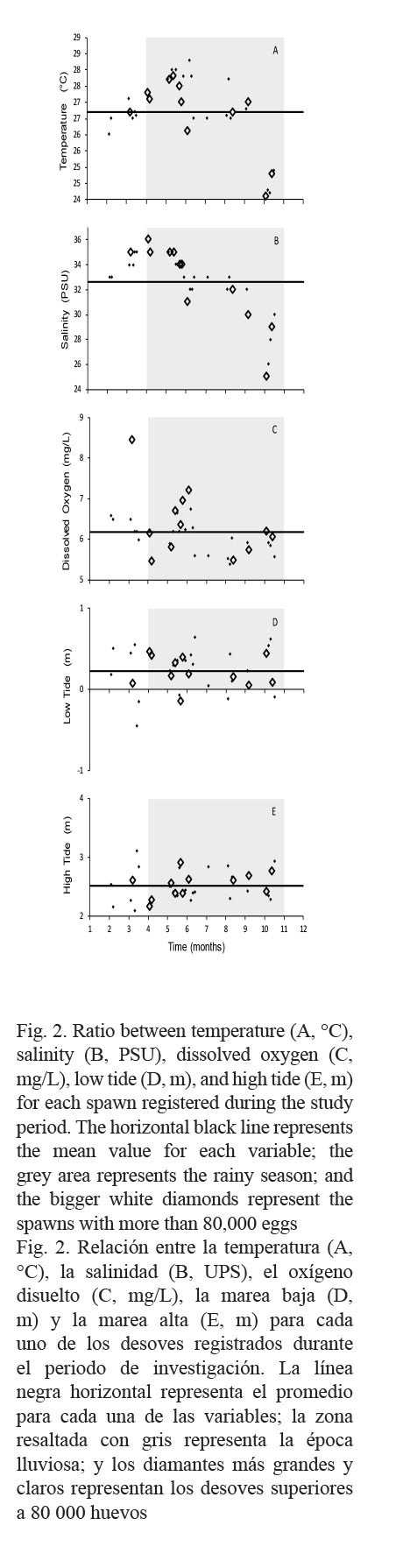
Although significant correlations were not obtained in the measured variables, several trends were observed (Fig. 1). The majority of the spawns coincided with tidal amplitudes. In most cases, an increase in precipitation was observed before each spawning. Spawns with the highest number of eggs occurred in May and August coinciding in both cases with the full moon and average tidal amplitude. Although correlations were observed between salinity-temperature (P ˂ 0.05, 0.556) and salinity-precipitation (P ˂ 0.05, - 0.544), there was no significant correlation of these variables with the number of spawned eggs and the fertilization percentage, or with the production of juveniles. A significant correlation was determined between the number of spawned eggs and fertilization percentage (P ˂ 0.05, 0.883), number of spawned eggs and production of juveniles (P ˂ 0.05, 0.904), and fertilization percentage and alevin production (P ˂ 0.05, 0.911).
Most spawns occurred during the rainy season (shaded area, Figs. 1 and 2), at temperatures higher than average (black line, Fig. 2A). A similar behavior was observed regarding salinity, with a tendency of a salinity level higher than average (black line, Fig. 2B). Regarding dissolved oxygen, most spawns were below the estimated average (black line, Fig. 2C). As far as low tides, most of the spawns tend to be distributed around average low tides (black line, Fig. 2D), while some others occurred during very low tides. More homogeneity was observed for average high tides, where spawns occurred closer to the average high tide (black line, Fig. 2E).
A total of 8,050 juveniles were produced from 1,958.431 fertilized eggs, which represented a 0.41% survival at the end of the larval rearing period (45 days).
DISCUSSION
Maturation in captivity and control of spawning were highly rated within the criteria evaluated for the selection of species with mariculture and restocking potential, being species with voluntary spawning the ones with the highest potential, followed by species that must be induced and have natural fertilization (Álvarez-Lajonchére & Ibarra-Castro, 2013).
Spontaneous spawning of wild weakfish C. squamipinnis was observed after two years in captivity under a constant regime of temperature (29 ± 1°C) (Boza et al. 2016a). Juveniles produced from these spawns were maintained under laboratory conditions. Similar to what happened with wild broodstock, the first generation of hatchery-produced weakfish completed vitellogenesis in October 2010 and began spontaneous spawning in March 2011.
Few croaker species reproduced in captivity have managed to adapt to captivity conditions and spontaneous spawning. Sciaenops ocellatus has been spontaneously spawned in captivity on the Southeast coast of the United States and in other regions since 1976, when the species entered the restocking program in that country (Thomas et al. 1995). The aquaculture of this species benefited from the restocking program, and reproduction techniques extended to other countries such as Martinique, China, Israel and Ecuador (Tucker, 1998; Cárdenas, 2011; 2012).
C. nebulosus also entered the Texas restocking program in the 1970s, and, although it has been reported to spawn voluntarily in captivity for several months under very good conditions, in the end hormonal induction was also applied (Tucker, 1998). Atractoscion nobilis was considered an endangered species in California and was, therefore, included in the restocking program. It was reported to spawn voluntarily in the tanks, but was also induced to ovulate. The orangemouth corvina, C. xanthulus, spawned spontaneously in tanks and was catalogued as having great potential for aquaculture (Thomas et al., 1995; Tucker, 1998). Recently, the species A. regius, distributed along the estuaries of the Mediterranean Sea and the Atlantic Coast of Europe and Africa, spawned spontaneously in Greece. Broodstock used in this experiment came from eggs reared in captivity, and 7 spawns were registered during 15 days (Mylonas et al. 2013a; b). However, in other locations where A. regius has been reproduced in captivity, spawning has been obtained by hormone treatment. Despite the excellent condition of the rest of the Sciaenidae species for cultivation in marine farms, their spawning needs to be induced through hormonal treatments (Tucker, 1998; Thomas et al. 1995; Cárdenas, 2011; 2012; Mylonas et al. 2013a; b). Consequently, weakfish, C. squamipinnis, had the advantage, over the other Sciaenidaes species, of having spontaneous spawning with enough fertilized eggs, guaranteeing a sustained supply of juveniles.
In most of the studied species, temperature and photoperiod are manipulated to simulate the changes that naturally occur so that fish mature in captivity (Thomas et al., 1995). Tropical species are not exposed to extreme changes of temperature and photoperiod, although it is has been verified that maintaining a constant temperature throughout the process facilitates maturation and spawning, as was the case with C. squamipinnis (Boza-Abarca et al. 2016a). In this case, spawning synchronization seemed to be more related to the tides and moon phases, as well as to certain precipitation events that occurred some days before spawning. This aspect should be taken into consideration when reproducing croaker species in the tropics in order to try to simulate both variables in tanks. In this study, there was a tendency to relate spontaneous spawning with moon phases and tidal amplitudes; however, no significant correlations were obtained between these variables.
Ovarian biopsies of broodstock reared in the laboratory determined oocytes ranging between 0.4 to 0.6 mm in diameter ready to move to the final maturation and spawning phase. The average weight of these specimens was 339.45 ± 79.89 g, with a total length of 33.93 ± 2.93 cm. In studies conducted with monthly weakfish samples captured in the Gulf of Nicoya, the estimated size at first maturity (SFM) was 40 cm in 1988 (Campos, 1992), while in 1999 size at first maturity decreased to 34 cm (Vásquez, 1999), which was later confirmed by Soto et al. (2003) and Robles (2007) in the Panamanian Pacific. This shows a decrease of at least 6 cm in length at first maturity (Marín & Vásquez, 2012). In other species, size at first maturity is reached at lengths greater than those observed in this study. Size ranges between 70-107 cm in A. japonicus, depending on the study site, with an approximate age of 5-6 years; length varies between 60-86 cm (4-5 years) in A. regius; the estimated size of wild fish in S. ocellatus was 60-75 cm (3-6 years); although it was similar in fish reared in the Laboratory, the time to reach this length was two years, while S. umbra reaches the size at first maturity at 20-30 cm (Cárdenas, 2011). It has been observed that size at first maturity is reduced when fish are reared in captivity; however, this is not the case of the weakfish, since the SFM found in this study is similar to the one estimated by several authors in the Gulf of Nicoya (Vásquez, 1999; Soto et al. 2003). In terms of the age for the weakfish to reach maturation in captivity, it took two years from the detection of the first vitellogenic eggs to spawning.
The first study that reported a spontaneous spawning in the A. regius species establishes a minimum of 4 years under optimum conditions for a successful spawning to occur (Mylonas et al. 2013a). In the case of C. squamipinnis, the two years that the adaptation process took for the samples captured in the Gulf of Nicoya to spawn (Boza-Abarca et al. 2016a in press), and the ones reared in the laboratory from egg indicate that the species adapted well to the captivity conditions in the Juan Bertoglia Richards Marine Biology Station.
CONCLUSIONS
A total of 34 spawns were observed during the period from March to October 2011 for a total of 2,853.799 eggs, of which 1,958.431 eggs were fertile, producing 8,050 second-generation juveniles. Weakfish C. squamipinnis is one of the few species that spawns spontaneously in captivity at an average length of 33.93 ± 2.93 cm and an average weight of 339.45 ± 79.98 g. The period from hatching until the detection of the first specimens evidencing maturation was 2 years. Spontaneous spawning could be related to the crescent, full, and new moon phases and, therefore, to a greater tidal amplitude, although no significant correlations were observed between these variables. In most spawns a slight precipitation was observed before it occurred and, in these cases, a premonitory spawning was detected before the main spawning. The size at which broodstock reared in captivity matured was similar to the size at first maturity estimated for fish caught in the Gulf of Nicoya, being close to 34 cm. The results obtained in this study confirm that the C. squamipinnis species is a strong candidate in possible restocking programs and mariculture projects as a productive alternative for fishermen.
ACKNOWLEDGMENTS
This study was financed by Law on Tuna No. 6267 and the School of Biological Sciences at Universidad Nacional de Costa Rica. The authors would like to thank Captain Orlando Torres for collecting and transporting the fish from the cages to the Juan Bertoglia Richards Marine Biology Station. We also wish to thank the anonymous reviewers for their detailed review of the manuscript, which contributed to enrich the information presented.
BIBLIOGRAPHY
Álvarez-Lajonchére, L. & Ibarra-Castro, L. (2013). Aquaculture species selection method applied to marine fish in the Caribbean. Aquaculture, 408-409, 20-29. http://dx.doi.org/10.1016/j.aquaculture.2013.05.020
Ballagh, A. D., Pankhurst, P. M. & Fielder, D. S. (2011). Embryonic development of mulloway, Argyrosomus japonicus, and egg surface disinfection using ozone. Aquaculture, 318, 475-478. http://dx.doi.org/10.1016/j.aquaculture.2011.06.005
Bennetti, D. D., Refik, M. O., Zink, I., Cavalin, F. G., Sardenberg, B., Palmer, K., Denlinger, B., Bacoat, D. & O´Hanlon, B. (2007). Aquaculture of Cobia (Rachycentron canadum) in the Americas and the Caribbean. In I. C. Liao & E. M. Leaño (Eds.), Cobia Aquaculture: Research, Development, and Commercial Production (pp. 57-78). Asian Fisheries Society, Manila, Philippines, World Aquaculture Society, Louisiana, USA: The Fisheries Society of Taiwan, Keelung, Taiwan, and National Taiwan Ocean University, Keelung, Taiwan.
Boza-Abarca, J., Calvo-Vargas, E., Solís-Ortiz, N. & Komen, J. (2008). Desove inducido y crecimiento larval del pargo manchado, Lutjanus guttatus, en la Estación de Biología Marina de Puntarenas, Costa Rica. Cienc. Mar., 34, 239-252.
Boza-Abarca, J., Valverde-Chavarría, S., Calvo-Vargas, E., Ramírez-Alvarado, M. & Rodríguez-Gómez, E. (2011). Hormone-induced spawning of wild and captive-grown spotted rose snapper Lutjanus guttatus using carp pituitary suspension and human chorionic gonadotropin. Cienc. Mar., 37(2), 125-139. http://dx.doi.org/10.7773/cm.v37i2.1802
Boza-Abarca, J., Ramírez-Alvarado, M., Barquero-Chanto, J., Calvo-Vargas, E. & Berrocal-Artavia, K. (2016a). Desove espontáneo, ontogenia y crecimiento en cautiverio de Cynoscion squamipinnis (Perciformes: Sciaenidae). Rev. Biol. Trop., 64(3), 991-1005.
Boza-Abarca, J., Ramírez-Alvarado, M., Barquero-Chanto, J., Calvo-Vargas, E. & Berrocal-Artavia, K. (2016b). Crecimiento de juveniles de la corvina aguada, Cynoscion squamipinnis criados en cautiverio. UNICIENCIA, 30(2), 63-74. http://dx.doi.org/10.15359/ru.30-2.5
Campos, J. (1992). Estimates of length at first sexual maturity in Cynoscion spp. (Pisces: Sciaenidae) from Gulf of Nicoya, Costa Rica. Rev. Biol. Trop., 40(2), 239-241.
Cárdenas, S., Duncan, N., Pastor, E., Fernández-Palacios, H., Rodríguez-Rúa, A., Estévez, A., Grau, A. & Schuchardt, D. (2008, September). Meagre (Argyrosomus regius) broodstock management in the Spanish R&D Project PLANACOR (JACUMAR). Paper presented at the International Conference on Aquaculture Europe 2008, European Aquaculture Society, Special Publication N° 37, Krakow, Poland.
Cárdenas, S. (2011). Cultivo de corvina (Argyrosomus regius). Cuadernos de acuicultura. Madrid, España: Fundación Observatorio Español de Acuicultura.
Cárdenas, S. (2012). Biología y acuicultura de corvinas en el mundo. AquaTIC, 37, 1-13.
Chacón, A., Araya, H., Vásquez, A., Brenes, R., Marín, B., Palacios, J., Soto, R., Mejía-Arana, F., Shimazu, Y. & Hiramatsu, K. (2007). Estadísticas Pesqueras del Golfo de Nicoya, Costa Rica, 1994-2005. Proyecto Manejo Sostenible de la Pesquería para el Golfo de Nicoya. Puntarenas, Costa Rica: JICA-UNA-INCOPESCA.
Duncan, N., Francesc, P., Aguilera, C., Montero, F. E., Norambuena, F., Carazo, I., Carbo, R. & Estévez, A. (2007a, January). Domestication and GnRHa induced-spawning of meagre (Argyrosomus regius). Paper presented at the 8th International Symposium on Reproductive Physiology of Fish, Saint Malo, France.
Duncan, N. J., Estévez, A. & Mylonas, C. C. (2007b, setiembre). Efecto de la inducción hormonal mediante implante o inyección de GnRHa en la cantidad y calidad de puestas de corvina (Argyrosomus regius). Paper presented at XI Congreso Nacional de Acuicultura “Cultivando el Futuro”, Vigo, España.
Duncan, N., Estévez, A., Padros, F., Aguilera, C., Montero, F. E., Norambuena, F., Carazo, I., Carbo, R. & Mylonas, C. C. (2008). Acclimation to captivity and GnRHa-induced spawning of meagre (Argyrosomus regius). Cybium Int. J. Ichtyol., 32, 332-333.
Duncan, N., Estévez, A., Porta, J., Carazo, I., Norambuena, F., Aguilera, C., Gairin, I. & Bucci, F. (2012). Reproductive development, GnRHa-induced spawning and egg quality of wild meagre (Argyrosomus regius) acclimatised to captivity. Fish. Physiol. Biochem., 38, 1273-1286. http://dx.doi.org/10.1007/s10695-012-9615-3
Duncan, N. J., Estévez, A., Fernández-Palacios, H., Gairin, I., Hernández-Cruz, C. M., Roo, F. J., Schuchart, D. & Vallés, R. (2013). Aquaculture production of meagre (Argyrosomus regius), hatchery techniques, ongrowing and market. In G. Allan & G. Burnell (Eds.), Advances in Aquaculture Hatchery Technology (pp. 519-541). Cambridge, UK: Woodhead Publishing Limited.
Gamzis, K. & Neke, M. (2008, July). Embryonic development stages of meagre Argyrosomus regius Asso 1801 under rearing conditions. Paper presented at 8̊ Larval Biology Symposium, Lisboa, Portugal.
García-Alonso, J. & Vizziano, D. (2004). Induction of oocyte maturation in the White croaker Micropogonias furnieri (Pisces: Sciaenidae) by human Chorionic gonadotropin. Braz. J. Biol., 64, 73-80. http://dx.doi.org/10.1590/S1519-69842004000100009
Jiménez, M. T., Pastor, E., Grau, A., Alconchel, J. I., Sánchez, R. & Cárdenas, S. (2005). Revisión del cultivo de esciénidos en el mundo, con especial atención a la corvina Argyrosomus regius (Asso, 1801). Bol. Inst. Esp. Oceanogr., 21, 169-175.
Koumoundouros, G., Kouttouki, S., Georgakopoulou, E., Papadakis, I., Maingot, E., Kaspiris, E., Kiriakou, Y., Georgiou, G., Divanach, P., Kentouri, M. & Mylonas, C. (2005). Ontogeny of the shi drum Umbrina cirrosa (Linnaeus 1758), a candidate new species for aquaculture. Aquacult. Res., 36, 1265-1272. http://dx.doi.org/10.1111/j.1365-2109.2005.01314.x
López, L. M. & Durazo, E. B. (2008). Guía para la Nutrición y Cultivo de Corvina Blanca. Ensenada, B. C., México: Grupo de Nutrición, Universidad Autónoma de Baja California, Facultad de Ciencias Marinas.
Marín, B. E. & Vásquez, A. R. (2012). Estimación de la talla de primera madurez sexual criterio L50% (TPM) de la corvina reina Cynoscion albus (Perciforme: Sciaenidae) bajo condiciones de sobreexplotación de su población en el Golfo de Nicoya, Costa Rica. (Technical Document N° 11). Puntarenas, Costa Rica: Instituto Costarricense de Pesca y Acuicultura.
Musson, M. & Kaiser, H. (2014). Development of the digestive system in dusky kob, Argyrosomus japonicus. Aquacult. Int., 22, 783-796. http://dx.doi.org/10.1007/s10499-013-9706-x
Mylonas, C. C., Mitrizakis, N., Papadaki, M. & Sigelaki, I. (2013a). Reproduction of hatchery-produced meagre Argyrosomus regius in captivity I. Description of the annual reproductive cycle. Aquaculture, 414-415, 309-317. http://dx.doi.org/10.1016/j.aquaculture.2013.09.009
Mylonas, C. C., Mitrizakis, N., Castaldo, C. A., Cerviño, C. P., Papadaki, M. & Sigelaki, I. (2013b). Reproduction of hatchery-produced meagre Argyrosomus regius in captivity II. Hormonal induction of spawning and monitoring of spawning kinetics, egg production and egg quality. Aquaculture, 414-415, 318-327. http://dx.doi.org/10.1016/j.aquaculture.2013.09.008
Papadakis, I. E., Kentouri, M., Divanach, P. & Mylonas, C. (2013). Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture, 388-391, 78-88. http://dx.doi.org/10.1016/j.aquaculture.2013.01.012
Robles, Y. (2007). Análisis biológico pesquero de pargos y corvinas en el Golfo de Montijo, Veraguas, Panamá. Unpublished Master Thesis, Universidad de Panamá, Panama City, Panama.
Schiavone, R., Zilli, L., Storelli, C. & Vilella, S. (2012). Changes in hormonal profile, gonads and sperm quality of Argyrosomus regius (Pisces, Scianidae) during the first sexual differentiation and maturation. Theriogenology, 77, 888-898. http://dx.doi.org/10.1016/j.theriogenology.2011.09.014
Soto, R., Mejía-Arana, F. & Hiramatsu, K. (2003). Estimación de la longitud total para la primera madurez en corvina aguada, y pargo mancha en el Golfo de Nicoya, Costa Rica. (Informe corte 2). Puntarenas, Costa Rica: Proyecto Manejo Sostenible de las Pesquerías del Golfo de Nicoya, Costa Rica.
SPSS. (2006). Statistical Package for the Social Science for Windows, version 17.0. Chicago, USA: SPSS Inc.
Thomas, P., Arnold, C. R. & Holt, G. (1995). Red Drum and other Sciaenids. In N. R. Bromage & R. J. Roberts (Eds.), Broodstock management and egg and larval quality (pp. 118-137). Oxford, Great Britain: Blackwell Science.
Tucker, J. W. Jr. (1998). Marine Fish Culture (1st ed.). Dordrecht, Netherlands. Kluwer Academic Publishers. http://dx.doi.org/10.1007/978-1-4615-4911-6
Valverde, S. & Boza, J. A. (1999). Inducción al desove en hembras del pargo mancha, Lutjanus guttatus (Steindachner 1869). UNICIENCIA, 15-16, 65-69.
Vásquez, A. R. (1999). Aspectos de la biología reproductiva de la corvina aguada (Cynoscion squamipinnis) en el Golfo de Nicoya, Costa Rica. Unpublished Licenciate Thesis, Universidad Nacional de Costa Rica, Heredia, Costa Rica.

Revista Ciencias Marinas y Costeras está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.