
Rev. Ciencias Veterinarias, Vol. 39, N° 2, [1-8], E-ISSN: 2215-4507, julio-diciembre, 2021
DOI: https://doi.org/10.15359/rcv.39-2.3
URL: http://www.revistas.una.ac.cr/index.php/veterinaria/index
Nosemosis in Africanized Honey Bee Colonies (Apis mellifera) in the Tropical Conditions of Costa Rica: Nosema apis or Nosema ceranae
Nosemosis en colmenas de abejas africanizadas (Apis mellifera) en las condiciones tropicales de Costa Rica: Nosema apis o Nosema ceranae
Nosemose em colméias de abelhas africanizadas (Apis mellifera) nas condições tropicais da Costa Rica: Nosema apis ou Nosema ceranae
Rafael A. Calderón1 ; Luis A. Sánchez2
; Luis A. Sánchez2
1- Programa Integrado de Patología Apícola, Centro de Investigaciones Apícolas Tropicales, Universidad Nacional, Heredia, Costa Rica. *Corresponding author. E-mail: rafael.calderon.fallas@una.cr
2- Programa Integrado de Ecología y Polinización, Centro de Investigaciones Apícolas Tropicales, Universidad Nacional, Heredia, Costa Rica. E-mail: luis.sanchez.chaves@una.cr
 Autor de correspondencia: rafael.calderon.fallas@una.cr
Autor de correspondencia: rafael.calderon.fallas@una.cr
Received: March 4, 2021 Corrected: April 19, 2021 Accepted: May 21, 2021
Abstract
The presence of nosemosis in Africanized honey bees in Costa Rica was studied. A total of 75 samples of adult bees from different country regions were selected for molecular diagnosis of nosemosis. Prior to PCR tests, Nosema spp. spores were morphologically identified in most of the bee samples using a light microscopy at 40x magnification. According to molecular analyses, most of the bee samples were found to be infected with Nosema ceranae. However, colonies showed no clinical signs of infection at any time during the sampling period, none of them being infected with Nosema apis. Surprisingly, 29.3% of the bee samples tested PCR negative to nosemosis. The origin of the bee samples collected from apiaries located in four of the seven provinces of Costa Rica showed the microsporidium is widely spread throughout the main beekeeping areas of the country. The pathological consequences of N. ceranae in Africanized honey bee colonies have not been well determined. Because of reports of honey bee colony losses in Europe related to microsporidian infections, the virulence of N. ceranae in Africanized honey bees needs to be studied.
Keywords: Nosema apis, Nosema ceranae, nosemosis, Africanized honey bees, Costa Rica
Resumen
Se estudió la presencia del microsporidio Nosema spp. en colmenas de abejas africanizadas en Costa Rica. Se seleccionaron un total de 75 muestras de abejas adultas de diferentes zonas apícolas del país, para el diagnóstico molecular de nosemosis. Previamente a la prueba de PCR, las esporas de Nosema spp. se identificaron morfológicamente con el microscopio de luz a un aumento de 40x. Con base en el análisis molecular, se determinó que la mayoría de abejas estaban infectadas con Nosema ceranae, aun cuando las colmenas no mostraban signos clínicos de la infección durante el periodo de muestreo. Por otra parte, ninguna de las muestras estaba infectada con Nosema apis. Un hallazgo para resaltar es que un 29.3% de las muestras de abejas resultaron negativas a nosemosis mediante el examen de PCR. El origen de las abejas, las cuales se colectaron de apiarios ubicados en cuatro de las siete provincias de Costa Rica, indica que el microsporidio N. ceranae está ampliamente distribuido en las principales zonas apícolas del país. Aún no se conoce con exactitud las consecuencias patológicas de la presencia de N. ceranae en colmenas de abejas africanizadas. Sin embargo, debido a la pérdida de abejas melíferas reportada en Europa, relacionada a infecciones de microsporidios, la virulencia de N. ceranae en abejas africanizadas debe ser estudiada.
Palabras claves: Nosema apis, Nosema ceranae, nosemosis, abejas africanizadas, Costa Rica
Resumo
Foi estudada a presença do microsporídio Nosema spp. em abelhas africanizadas na Costa Rica. Um total de 75 amostras de abelhas adultas de diferentes regiões do país foram selecionadas para o diagnóstico molecular de nosemose. Antes dos testes de PCR, os esporos de Nosema spp. foram identificados morfológicamente na maioria das amostras de abelhas usando microscopia de luz com aumento de 40x. De acordo com análises moleculares, a maioria das amostras de abelhas estavam infectadas com Nosema ceranae. No entanto, as colônias não apresentaram sinais clínicos de infecção em nenhum momento durante o período da amostragem. Por outro lado, nenhuma das amostras estavam infectadas com Nosema apis. É válido ressaltar que 29,3% das amostras de abelhas testaram negativo no exame de PCR para nosemose. A origem das amostras das abelhas coletadas em apiários localizados em quatro das sete províncias da Costa Rica mostrou que o microsporídio está amplamente distribuído nas principais áreas apícolas do país. As consequências patológicas de N. ceranae em colônias de abelhas africanizadas ainda não é bem conhecida. Entretanto, devido a relatos de perdas de colônias de abelhas na Europa relacionadas a infecções por microsporídios, a virulência de N. ceranae em abelhas africanizadas precisa ser estudada.
Palavras-chave: Nosema apis, Nosema ceranae, nosemose, abelhas africanizadas, Costa Rica
Introduction
Nosemosis is the most widespread of adult bee diseases causing significant economic losses to beekeepers (Giersch et al., 2009). Honey bees, Apis mellifera (Hymenoptera: Apidae), are parasitized by two microsporidians, Nosema apis (Zander) (nosemosis type A) and Nosema ceranae (Fries) (nosemosis type C) (Fries et al., 1996). Both species of microsporidae infect the epithelial layer of the ventriculus and midgut of adult bees, causing digestive disorders and shortening the life span of bees, with a resulting decrease in bee population (Higes et al., 2006; Huang et al., 2007). In the case of N. ceranae, some studies suggest this microsporidium occurs in other tissues as well (Chen & Huang, 2010).
N. apis was the first described microsporidium in honey bees (Ritter, 2001). It is known to have infected honey bees worldwide (Matheson, 1993). N. ceranae was first reported in colonies of the Asian hive bee, A. cerana (Fries et al., 1996), but it was also found in A. mellifera colonies in both Taiwan and Europe (Higes et al., 2006). Recently, a new species, Nosema neumanni was found in Uganda (Chemurot et al., 2017).
Previously, nosemosis in Africanized honey bees (AHB) was attributed exclusively to N. apis (Matheson, 1993). However, it appears N. ceranae is an emerging pathogen that has increased its distribution in the past decade by jumping from Asian honey bees A. ceranae to A. mellifera worldwide (Chen et al., 2008; Higes et al., 2008; Klee et al., 2007). The presence of N. ceranae in Africanized bees in Costa Rica was confirmed in 2006 (Calderón et al., 2008). Nevertheless, little data are available about the spread of N. ceranae in the country (Calderón & Ramírez, 2013). Some studies have described N. ceranae to have a higher prevalence than N. apis in A. mellifera, although both are widely distributed (Paxton et al., 2007). However, studies of Nosema spp. prevalence in Africanized honey bees in Costa Rica are scarce (Calderón & Ramírez, 2013). Furthermore, the pathological effects of N. ceranae in Africanized bees are not well understood, but due to reports of colony population depletion and high mortality in honey bee colonies connected to microsporidian infections (Higes et al., 2006), the virulence of N. ceranae in Africanized bees needs to be investigated.
The diagnosis of nosemosis has been made by detecting spores of Nosema spp. through microscopic analyses (Shimanuki & Knox, 2000). Nevertheless, spores produced by the two Nosema species are quite similar and very difficult to distinguish using light microscopy analysis. With the finding both N. ceranae and N. apis affect western honey bees (A. mellifera), proper molecular techniques are required to differentiate between these different species of microsporidia, because the spores of the two Nosema species cannot be really differentiate by their morphology (Fries et al., 1996). In addition, microscopic analyses are not as sensitive to detecting low levels of Nosema infection as molecular methods, such as PCR can be. Molecular techniques have been developed to improve accurate diagnoses of Nosema disease in the laboratory (Cueto et al., 2020; Higes et al., 2006; Klee et al., 2007; Martin-Hernández et al., 2007; Paxton et al., 2007). Here, we used PCR to characterize infections or co-infections by these two Nosema species in Africanized honey bee colonies. Prior to PCR analysis, spores were visually detected in most of the adult bee samples using light microscopy.
Materials and Methods
In this study, 532 adult bee samples of Africanized honey bee colonies (Spivak, 1991) from different areas of Costa Rica were randomly collected for Nosema spp. diagnosis. Most bee samples were collected from colonies with no clinical signs of the disease. Interestingly, reduced honey production had been reported by beekeepers in some of the sampled areas over the previous years.
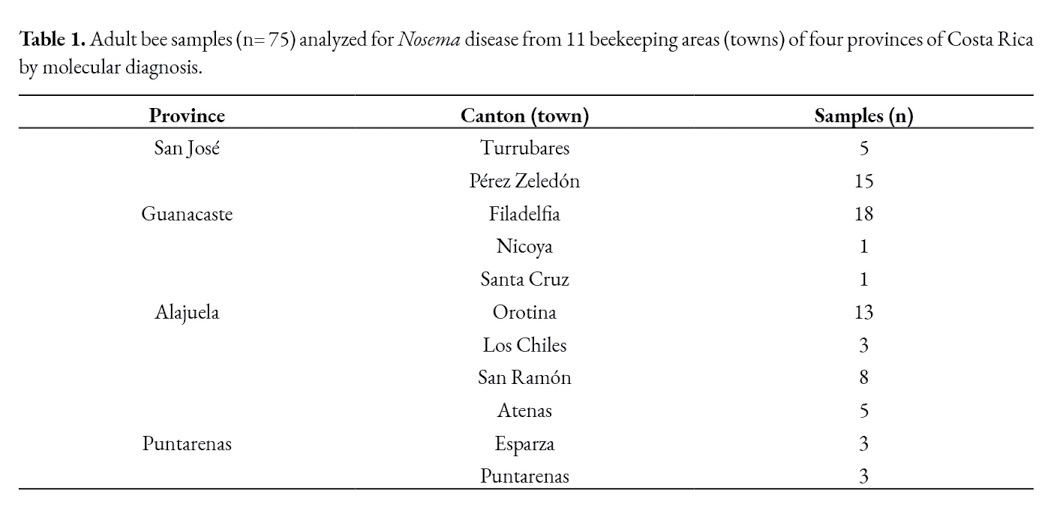
Foragers and adult bees from the brood nest were collected for Nosema spp. analysis. Gathering the foragers implied closing the hive entrance for 30 minutes and collecting them as they arrived. Furthermore, a comb from the brood nest was used to collect adult bees inside the colony (considered to be representative of the house bees). Samples of at least 100 bees were collected per colony. Preliminary analysis was made at the Bee Pathology Lab of the Tropical Beekeeping Research Center (CINAT), Heredia, Costa Rica, to select positive and suspected samples to Nosema spp. The abdomens of thirty adult bees from each colony were macerated in 30 ml of distilled water and microscopically examined for the presence of Nosema spp. spores. The spores were identified under the coverslip using a light microscopy at 40x (400x). All bee samples belonged to Africanized colonies naturally infected with Nosema ssp., showing diverse infection levels. The spore concentration was determined by counting with a haemocytometer (Neubauer chamber). Once the presence of microsporidian spores was confirmed (high infection levels) or suspected (spore concentrations were found at very low levels), 75 samples from apiaries located in four out the seven provinces of Costa Rica (Table 1) were selected for molecular diagnosis (from the 217 positive ones).
The molecular test (duplex PCR assay) was made at the Bee Pathology Lab of the Regional Beekeeping Center, Marchamalo, Spain. Honey bee samples were washed in distilled water; then, they were macerated with 5.0 ml of distilled water in a Stomacher 80 Biomaster (Seward) using bags with filters (BA6040/STR, Seward) for 2 minutes at medium speed in distilled water (PCR grade). The macerate was recovered in a tube and then centrifugated at 1200 rpm for 6 min. After centrifugation, the supernatant was discarded, and the pellet was resuspended 1.0 ml of ultrapure water (PCRq). The final suspension was shaken 30 times per s for 4 min with 0.1 g of glass beads (2 mm diameter) in a TissueLyser (Qiagen). Subsequently, 150 ul of the homogenate was incubated overnight at 56°C with 20 ul of proteinase K and 30 ul of ATL buffer (Qiagen), and DNA was extracted using a BioSprintTM 96 DNA Blood Kit (384: Qiagen, cat. No. 940057) in a Biosprint 96 robot (tissue protocol Qiagen). The DNA extracts were analyzed by duplex PCR using a Mastercycler ep gradient S (Eppendorf), and each PCR product was analyzed in a QIAxcel System (Qiagen), using a QIAxcel DNA High-Resolution Kit (Qiagen, No. 929002). PCR-specific primers for a region of the 16S rRNA gene of Microsporidia to detect both N. apis and N. ceranae were used. The PCR products were sequenced and compared to GenBank entries as described by Martin-Hernandez et al. (2007).
Results
A total of 75 samples of Africanized honey bees from different regions of Costa Rica were selected for molecular diagnosis. Prior to PCR, spores were identified and counted using light microscopy. The mean spore count (Nosema loads) varied between the brood nest and forager bees (Table 2).

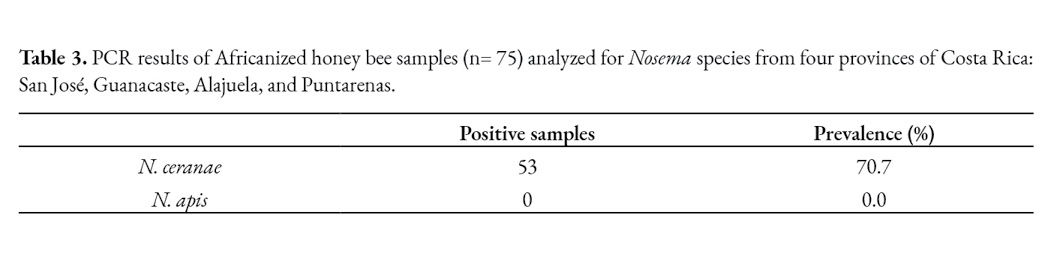
Based on the molecular analyses, most of the bee samples were found to be infected with N. ceranae, although colonies showed no clinical signs of infection at any time during the sampling period, none of them being infected with N. apis (Table 3). Surprisingly, it was observed 29.3% of the bee samples tested PCR negative to nosemosis.
The origin of the positive bee samples collected from different apiaries located in four out of the seven provinces of Costa Rica showed the microsporidium is distributed throughout the main beekeeping areas of the country.
Discussion
Given the economic and health impact on nosemosis in managed honey bee colonies, it is essential to know the spread and an accurate analysis of the epidemiological evolution of this illness under field conditions. N. ceranae was the only Nosema species identified in Africanized bees using the molecular technique throughout the study. It has been proposed that N. ceranae is more prevalent in warmer climates than N. apis (Fries, 2010). Furthermore, some reports currently suggest that N. ceranae is displacing N. apis in most regions worldwide (Moritz et al., 2010).
Although the presence of microsporidium spores was confirmed in most of the bee samples (low to high spore loads) using a light microscopy at 40x, it was found that one third of the bee samples tested PCR negative to nosemosis. These results reveal some variability in the detection of microsporidia depending on the random subsample analyzed, even when following OIE recommended sampling procedures. It means analysis of a single subsample collected at a specific time cannot provide conclusive information on the diagnosis or presence of Nosema spp. in the colony or apiary. It has to be considered that in nosemosis due to N. apis, the mean spore count per bee has traditionally been used to determine the extent of infection in a colony (Furgala & Hyser, 1969). A close relationship between spore count and the degree of infection has been established for this disease (Cornejo & Rossi, 1975); this parameter has been used to evaluate the need for treatment. However, the relationship between spore count and the colony’s health may not be so close. Indeed, the spore count is not directly related to the parasite burden and health status of whole colonies naturally infected by N. ceranae under field conditions (Higes et al., 2008).
Until 2006, it has been considered that Africanized colonies in Costa Rica were infected by one microsporidian, N. apis. The presence of N. ceranae in Africanized bees in this country was confirmed in 2006 (Calderon et al., 2008). At that time, using the genetic technique, all samples were found to be infected with N. ceranae. Nevertheless, a third part of the samples was infected with N. apis and N. ceranae together. In Brazil, among 637 samples analyzed from 10 states, between 2009 and 2012, 79.9% of the samples were infected with Nosema, of which 98.8% were N. ceranae and only 1.2% were N. apis (Texeira et al., 2013). In Mexico, Guzmán-Novoa et al. (2011) found 94,1% of the prevalence of N. ceranae and only 5.9% of N. apis in 32 samples from four different states. Some earlier observations of microsporidian infections in Africanized bees in Costa Rica may, in fact, have been observations of N. ceranae. How the microsporidium N. ceranae was introduced into the country is unknown, but it is most likely through the transport of honey bee queens, which beekeepers import from different countries. According to Klee et al. (2007), the world trade with honey bees, either by commercial or hobbyist beekeepers, is the main reason for the spread of N. ceranae.
The spore counts observed in this study are expected to be underestimates since most of them were conducted on bees collected from the brood combs (nurse or in-hive bees) that have lower spore counts than foraging bees (Higes et al., 2008). Although, Traver and Fell (2011) found no significant difference between in-hive and foragers in N. ceranae infection levels based on q-PCR results, which detects the earlier vegetative stage. In all samples, the mean spore count of foragers was higher than house bees. This result emphasized central comb samples are also unsuitable as indicators of the degree of infection of the colony. The results of this study are in agreement with the OIE recommendations that indicate that older bees must be sampled. Thus, foragers collected at the sealed entrance of the hive are a more reliable source for sampling in studies of nosemosis.
The pathological consequences of N. ceranae in Africanized colonies in Costa Rica are not well known. The variation in the spore count (low, moderate, high, and maximum spore count was obtained in foragers) was not associated with clinical signs of disease, and colonies were apparently as healthy as any other. Nevertheless, because of reports of colony losses of honey bees in Europe related to microsporidian infections (Higes et al., 2006), where N. ceranae is reported to be highly virulent (Paxton et al., 2007), the virulence of N. ceranae in Africanized bees needs to be studied. In Mexico, artificially infected workers (AHB) with two different spore concentrations of N. ceranae showed precocious foraging, reduced duration of foraging, and decreased in the longevity compared with non-infected ones (Fleites et al., 2018). Several undetermined factors might influence mortality rates, including spore storage, age of healthy uninfected newborn bees, host subspecies and parasitic strain (Botias et al., 2012).
In conclusion, according to these results N. ceranae is a more prevalent species in Africanized honey bee colonies in Costa Rica than N. apis and it is wide-spread throughout the country.
Acknowledgments
The authors thank the beekeepers for letting us inspect and sample their beehives for nosemosis detection. The authors are grateful to Dr. Mariano Higes and his research group for technical support and supervision during PCR analysis at the Bee Pathology Lab of the Regional Beekeeping Center, Marchamalo, Spain.
References
Botías, C., Martín, R., Meana, A. & Higes, M. (2012). Critical aspects of the Nosema spp. diagnostic sampling in honeybee (Apis mellifera L.) colonies. Parasitology Research, 110, 2557-2561. doi.10.1007/s00436-011-2760-2
Calderón, R. A., Sánchez, L. A., Yañez O. & Fallas N. (2008). Presence of Nosema ceranae in Africanized honey bee colonies in Costa Rica. Journal of Apicultural Research and Bee World, 47(4), 328-329. http://dx.doi.org/10.1080/00218839.2008.11101485
Calderón, R. A. & Ramírez, F. (2013). Enfermedades de las abejas melíferas, con énfasis en abejas africanizadas. EUNA. Heredia, Costa Rica
Chemurot, M., De Smet, L., Brunain, M., De Rycke, R. & De Graaf, D. (2017). Nosema neumanni (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. European Journal of Protistology, 61, 13-19. doi.10.1016/j.ejop.2017.07.002
Chen, Y., Evans, J. D., Smith, I. B. & Pettis J. S. (2008). Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. Journal of Invertebrate Pathology, 97(2), 186-188. doi:10.1016/j.jip.2007.07.010
Chen, Y. P. & Huang, Z. Y. (2010). Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie, 41, 364-374. https://doi.org/10.1051/apido/2010021
Cornejo, L. G. & Rossi, C. O. (1975). Enfermedades de las abejas, su profilaxis y prevención. Hemisferio Sur. Buenos Aires.
Cueto, S. A., López, G., Orozco, C., Gómez, S. D., Moreno, K., Espinoza, K. O., Guerrero, J. G., Silva, L. E., Trasviña, E. & Monge, F. J. (2020). Prevalence and geographical distribution of Nosema apis and Nosema ceranae in apiaries of Northwest of Mexico using a duplex real time PCR with melting curve. Journal of Apicultural Research, 59(2), 195-203. https://doi.org/10.1080/00218839.2019.1676999
Fleites, F., Quezada, J. & Medina, L. (2018). Onset of foraging and lifespan of Africanized honey bees (Apis mellifera) infected with different levels of Nosema ceranae spores in Neotropical Mexico. Apidologie, 49, 781-788. doi:10.1007/s13592‐018‐0602‐2
Fries, I., Feng, F., Da Silva, A., Slemenda, B. & Pieniazek, J. (1996). Nosema ceranae n. sp. (Microspora, Nosematidae). Morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis ceranae (Hymenoptera, Apidae). European Journal of Protistology, 32, 356-365. https://doi.org/10.1016/S0932-4739(96)80059-9
Fries, I. (2010). Nosema ceranae in European honeybees (Apis mellifera). Journal of Invertebrate Pathology, 103, S73-S79. https://doi.org/10.1016/j.jip.2009.06.017
Furgala, B. & Hyser, R. A. (1969). Minnesota Nosema survey. American Bee Journal 109, 460-461.
Giersch, T., Berg, T., Galea, F. & Hornitzky, M. (2009). Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie, 40, 117-123. doi:10.1051/apido/2008065
Guzman-Novoa, E., Hamiduzzaman, M., Arechavaleta-Velazco, M. E., Koleuglu, G., Valizadeh, P. & Correa-Benitez, A. (2011). Nosema cerenae has parasite Africanized honey bees in Mexico since at least 2004. Journal of Apicultural Research, 50(2), 167-169. doi:10.3896/IBRA.1.50.2.09
Higes, M., Martin, R. & Meana, A. (2006). Nosema ceranae, a new microsporidian parasite in honey bees in Europe. Journal of Invertebrate Pathology, 92, 93-95. doi:10.1016/j.jip.2006.02.005
Higes, M., Martín-Hernández, R., Botías, C., Garrido-Bailón, E., González-Porto, A. V., Barrios, L., Nozal, M., Bernal, J. L., Jiménez, J. J., García-Palencia, P. & Meana, A. (2008). How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology, 10, 2659-2669. doi: 10.1111/j.1462-2920.2008.01687.x
Huang, W. F., Jiang, J. H., Chen, Y. W. & Wang, C. H. (2007). A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie, 38, 30-37. https://doi.org/10.1051/apido:2006054
Klee, J., Besana, A. M., Genersch, E., Gisder, S., Nanetti, A., Tam, D. Q., Chinh, T. X., Puerta, F., Ruz, J. M., Kryger, P., Message, D., Hatjina, F., Korpela, S., Fries, I. & Paxton, R. J. (2007). Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. Journal of Invertebrate Pathology, 96, 1-10. doi:10.1016/j.jip.2007.02.014
Martin-Hernández, R., Meana, A., Prieto, L., Salvador, A. M., Garrido-Bailón, E. & Higes, M. (2007). Outcome of colonization of Apis mellifera by Nosema ceranae. Applied Environmental Microbiology, 73, 6331-6338. doi:10.1128/AEM.00270-07
Matheson, A. (1993). World bee health report. Bee World, 74, 176-212. https://doi.org/10.1080/0005772X.1993.11099183
Moritz, R., De Miranda, J., Fries, I., Le Conte, Y., Neumann, P. & Paxton R. (2010). Research strategies to improve honeybee health in Europe. Apidologie, 41, 227-242. https://doi.org/10.1051/apido/2010010
Paxton, R., Klee, J., Korpela, S. & Fries, I. (2007). Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie, 38, 558-565. https://doi.org/10.1051/apido:2007037
Ritter, W. (2001). Enfermedades de las abejas. Acribia S.A. Zaragoza.
Shimanuki, H. & Knox D. A. (2000). Diagnosis of honey bee diseases. Department of Agriculture, Agriculture Handbook.
Spivak, M. (1991). The Africanization process in Costa Rica. Westview Press Inc. Boulder, CO.
Texeira, E. W., Guimaraes, L., Sattler, A., Message, D., Teles, M. L., Fonseca, M., Lopes, M. & Mauricio, T. (2013). Nosema ceranae has been present in Brazil for more than three decades infecting Africanized honey bees. Journal of Invertebrate Pathology, 114, 250-254. doi:10.1016/j.jip.2013.09.002
Traver, B. & Fell, R. (2011). Prevalence and infection intensity of Nosema in honeybee (Apis mellifera L.) colonies in Virginia. Journal of Invertebrate Pathology, 107, 43-49. doi:10.1016/j.jip.2011.02.003
 Licencia Creative Commons Atribución-No-Comercial SinDerivadas 3.0 Costa Rica
Licencia Creative Commons Atribución-No-Comercial SinDerivadas 3.0 Costa Rica