
Rev. Ciencias Veterinarias, Vol. 34, N° 1, [39-49], ISSN: 2215-4507, enero-junio, 2016
DOI: http://dx.doi.org/10.15359/rcv.34-1.3
URL: http://www.revistas.una.ac.cr/index.php/veterinaria/index
Measurement of thyroid hormones and cortisol in horses with an automated immunoassay analyzer
Medición de hormonas tiroideas y cortisol en caballos mediante un analizador de inmunoensayo automatizado
Marcela Suárez-Esquivel1, Laura Castro-Ramírez1
1 Department of Physiology, School of Veterinary Medicine, Universidad Nacional, Costa Rica
marcela.suarez.esquivel@una.cr / laura.astro.ramirez@una.cr
Received: 17 February 2015. Corrected: 13 January 2016. Accepted: 17 February 2016.
Abstract: Even though thyroid gland diseases are unusual in horses, it is important to have a reference interval for thyroid hormones in this species at every veterinary endocrine laboratory. In addition, cortisol and thyroid hormone measurement is useful in horses, not only for disease diagnosis, but as a tool for monitoring and researching performance and metabolic rate.
The objectives of this study included (i) to test the capacity of the automated analyzer AIA 360® (TOSOH Bioscience) to measure thyroid hormones and cortisol in equine serum in a reliable way, and (ii) to establish a reference interval for thyroid hormones in Costa Rican horses using the AIA-360®.
A total of 68 healthy and treatment free horses (31 males and 37 females) were sampled. Horses were grouped into three different categories according to their ages. Serum concentrations of total thyroxine (TT4), free thyroxine (FT4), and cortisol were quantified with the AIA-360®. Statistic relationships of hormonal values, gender and age were determined using Kruskal-Wallis, Dunn and Wilcoxon (U Man-Whitney) tests. P <0.05 was considered significant.
Selected reference intervals were: TT4 14.16 – 46.33 nmol/L, FT4 3.60 – 16.09 pmol/L and cortisol 23.45 – 166.64 nmol/L. No statistically significant relationship was found between gender, age, and hormone values. Besides, significant relationships (p<0.05) were found between hormone levels and some environmental parameters. Animals living at higher altitudes showed higher cortisol levels, while cortisol decreases as the environmental temperature rises. Moreover, the FT4 levels are higher as precipitation increases. Finally, TT4 seems to be lower as day light length increases; however, no statistical relationship was found (p<0.30). Our results show that the use of the automated analyzer AIA-360® is suitable to measure thyroid hormones and cortisol, and that the values found are comparable with other studies and analysis techniques.
Keywords: horses, thyroid hormones, cortisol, reference interval, AIA-360®
Resumen: A pesar de que las enfermedades tiroideas son poco comunes en equinos, es importante contar con un intervalo de referencia para hormonas tiroideas en cualquier laboratorio de endocrinología veterinaria. Asimismo, la medición de cortisol y hormonas tiroideas es útil en caballos, no solamente en el diagnóstico de enfermedades, sino que, ambos tipos de hormonas, son utilizadas como herramientas para monitorización e investigación del desempeño físico y la tasa metabólica.
Los objetivos de este estudio son: (i) probar la capacidad del analizador automatizado AIA 360® (TOSOH Bioscience) para la medición de hormonas tiroideas y cortisol en suero equino de forma confiable y; (ii) establecer un intervalo de referencia para hormonas tiroideas en caballos costarricenses utilizando el AIA -360®.
Se muestreó 68 caballos saludables, libres de medicación (31 machos y 37 hembras) y se agruparon en tres categorías, con base en la edad. Las concentraciones séricas de tiroxina total (TT4), tiroxina libre (FT4) y cortisol fueron cuantificadas en el AIA-360®. La relación estadística, entre sexo, edad y valores hormonales, se determinó con las pruebas de Kruskal-Wallis, Dunn y Wilcoxon (U Man-Whitney). Se consideró significativo un valor de p <0.05.
Los intervalos de referencia fueron los siguientes: TT4 14.16 – 46.33 nmol/L, FT4 3.60 – 16.09 pmol/L y cortisol 23.45 – 166.64 nmol/L. Estadísticamente, no se encontró relación significativa entre el sexo, la edad y los valores hormonales. Se observó relaciones significativas (p<0.05) entre los niveles hormonales y algunos de los parámetros medio ambientales en los cuales se encontraban los animales. A mayor altitud, los niveles de cortisol fueron más elevados; mientras que, conforme aumentaba la temperatura ambiental, los valores de cortisol fueron más bajos. Los niveles de FT4 incrementaron a mayor precipitación. La concentración de TT4 tiende a disminuir conforme aumentan las horas-luz diarias. Sin embargo, no se encontró una relación estadística para ello (p<0.30). Los resultados muestran que el AIA-360® es útil para la medición de hormonas tiroideas y cortisol; además, que los valores encontrados son comparables con otros estudios y técnicas de análisis.
Palabras clave: equinos, hormonas tiroideas, cortisol, intervalo de referencia, AIA-30®.
Introduction
Equine thyroid gland diseases are reported as rare conditions (Hilderbran et al. 2014). However, some authors and clinicians consider that thyroid gland dysfunction in horses could often be misdiagnosed due to a poor understanding of equine thyroid physiology and because reference intervals for thyroid hormones differ considerably among laboratories and measurement techniques (Mendoza et al. 2013). In addition, thyroid hormones are important physiological indicators commonly used in studies concerning metabolic rate and general development (Medica et al. 2011).
Cortisol concentration measurement in serum is used as a way to test for pituitary pars intermedia dysfunction in horses (Dybdal et al. 1994), which is a naturally occurring, progressive neuroendocrine disease in aged horses, that puts them at high risk of developing laminitis, secondary infections, and metabolic disturbances (Schott 2002; Cordero et al. 2012). Also, elevated concentrations of cortisol in serum have been associated with colic presentation and the illness severity; consequently, cortisol levels may provide additional decision-making and prognostic information for horses with colic (Mair et al. 2014). For these reasons and the usefulness of this measurement as a metabolic and stress research tool, it is important to be able to determine cortisol concentrations in equine serum.
Hormones have commonly been measured in different sample matrixes (plasma, serum, feces, milk, fur, saliva) using the radioimmunoassay (RIA) method, which has been considered the “gold standard” technique for hormonal analysis. However, the use of radioactive isotopes requires licensing, storage facilities, and safety devices (Singh et al. 1997) that are not available in all laboratories or institutions. Recently, other measurement techniques have applied the RIA principles using non-radioactive reagents, as the chemiluminescent immunoassay (CLIA) and chemiluminescent-enzyme-immunoassay (CLEIA) methods (Wheeler 2013).
Currently, several automated immunoassay analyzers are available for veterinary medicine. Among them, the AIA- 360® (Tosoh Bioscience) has become available for veterinary analysis, and its use for thyroid hormones and cortisol measurement in dogs and cats has been previously documented (Suárez-Esquivel and Castro-Ramírez 2012; Higgs et al. 2014). This study is aimed at (i) testing the AIA-360® capacity to measure thyroid hormones (TT4 and FT4) and cortisol in equine serum in a reliable way, and (ii) establishing a reference interval for thyroid hormones in Costa Rican horses using the AIA-360®.
Materials and Methods
Animals
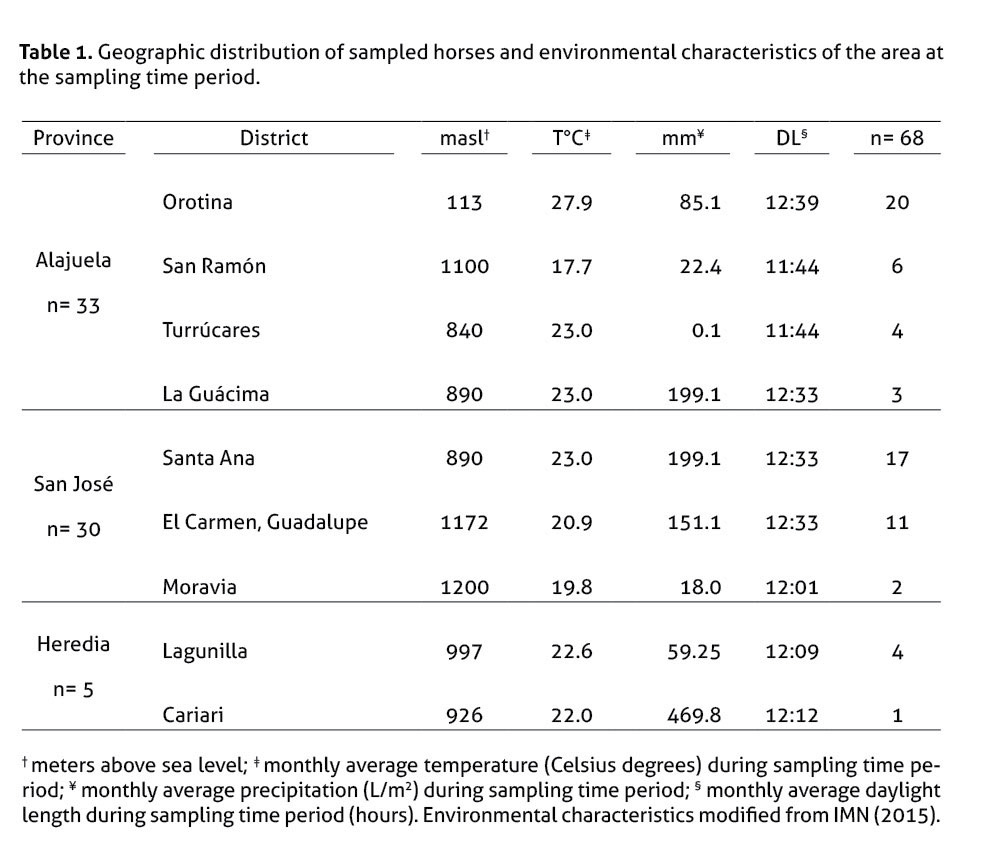
Sixty eight disease-free horses (31 males, 37 females) were used in the study. Animals were considered healthy based on a normal clinical history, physical examination, hematology and clinical biochemical evaluation. All the animals received suitable management conditions and were used with their owners’ consent. Animals receiving any kind of treatment were excluded from the study.
Out of the 68 horses, 35 were pure breed and 33 were mixed breed. Animals were grouped based on age frequency distribution (range: 3 months–26 years) into 3 groups: <5 years old (n = 15), 6–10 years old (n = 25) and >10 years old (n = 27).
The animals were sampled from 3 different provinces (table 1): Alajuela (n=33), San José (n=30), and Heredia (n=5).
Sample handling and hormone measurement
Blood samples were collected approximately from 9 to 11am, by jugular venipuncture and drawn into either a plain vacutainer tube for serum or into an ethylenediaminetetraacetic acid (EDTA) vacutainer tube. During blood collection, the animals received minimum handling, since no transportation and minimal physical restriction were involved in order to reduce handling stress. The blood samples without EDTA were centrifuged, and serum was separated. The serum samples were stored frozen at -20ºC for further endocrinological use.
Samples were evaluated for total thyroxine (TT4), free thyroxine (FT4) and cortisol quantification with the automated analyzer TOSOH® AIA-360, which uses a competitive fluorescent enzyme immunoassay, which runs in small, single-use test cups that contain all necessary reagents. Accuracy and performance data for human and canine T4 and cortisol, including analyte recovery and dilutional studies, had been previously evaluated (Higgs et al. 2014). Daily checks, calibration curves and maintenance procedures were performed as described in the System Operator’s Manual.
Animals were considered healthy based on hematological and biochemical analyses, which were performed on the same collection day at the Clinical Analysis Laboratory from the School of Veterinary Medicine, Universidad Nacional, following previously standardized-in-house protocols. Samples with pronounced hemolysis or lipemia were excluded.
Statistical analysis
InfoStat/E version 2014 (InfoStat) was used for median, average (X), and standard deviation (SD) calculation of the data. The reference intervals for TT4, FT4 and cortisol were generated using the P3 and P97 percentiles in the sampled population. Kruskal-Wallis tests were performed using InfoStat/E version 2014 (InfoStat) in order to determine the relationship between age or breed and the hormonal values. The Wilcoxon (U Man-Whitney) test was used for the comparison of hormonal values by gender. P<0.05 was considered significant.
The effects of environmental parameters such as altitude (meters above sea level), temperature (degrees Celsius), precipitation (L/m2) and day light length (hours) during the sampling period on the hormone levels were estimated using Spearman Correlations with parametric and non- parametric data.
Results
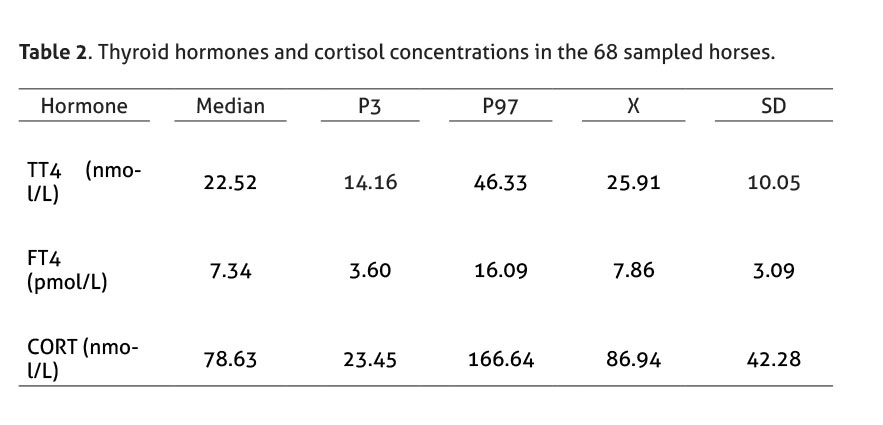
Hormonal concentrations of the 68 sampled horses (31 males and 37 females; average 9.7 years old) and descriptive statistical analysis are shown in Table 2.
Females’ TT4 and cortisol values were slightly higher than males’ (table 3); similarly, age group 2 (6-10 years) showed higher TT4 and FT4 levels (table 4). However, no statistical differences were found (p>0.05).


The selected reference interval for TT4 was 14.16 - 46.33 nmol/L, which is comparable to the range reported by several authors (Morris & García 1985; Meredith & Dobrinski 2004; Breuhaus et al. 2006; Graves et al. 2006; Kheirkhah & Hassanpour 2013; Mendoza et al. 2013; Hilderbran et al. 2014). In addition, the selected reference interval for FT4 was 3.60 - 16.09 pmol/L (Breuhaus et al. 2006; Graves et al. 2006; Mendoza et al. 2013). Besides, the selected reference range for cortisol, 23.45 – 166.64 nmol/L, coincides with previous reports using radioimmunoassay (Haritou et al. 2008; Mair & Sherlock 2009; Schenk et al. 2012; Liburt et al. 2013).
The analysis of environmental parameters on the hormone values revealed a significant effect (p>0.05) on the following correlations: i) animals at higher altitudes ranging from 113 to 1200 masl have higher levels of cortisol; ii) animals under warmer conditions (ranging 17.7-27.9°C) have lower cortisol values; iii) under higher precipitation conditions (ranging from 0.1 to 469.8 L/m2), animals show increased levels of FT4. We also found a slight correlation (p<0.3) between longer daylight lengths (ranging from 11:44 to 12:39 hours) and lower TT4 values.
Discussion
The levels of serum thyroid hormones may be affected by several endogenous factors (gender, age, breed, and nutrition) and external factors such as diseases, climate or exercise (Graves et al. 2006; Eshratkhah et al. 2010). The measurement of these hormonal levels is important in the diagnosis of thyroid disorders and their relationship with other alterations in hematological and biochemical states (Eshratkhah et al. 2010). Even though thyroid gland dysfunction is fairly uncommon in adult horses, some clinicians consider important to exclude thyroid malfunction as a main cause of clinical manifestations (Hilderbran et al. 2014). In addition, hypothyroidism in horses has been related to conditions such as laminitis, poor reproduction performance, and chronic myositis (Breuhaus et al. 2006). However, the information related to thyroid function in horses is scarce (Breuhaus et al. 2006), specially under tropical conditions.
Our results demonstrate that the use of the automated analyzer AIA-360® is suitable for thyroid hormone and cortisol measurement in horse serum, and the values found are comparable with other studies and analysis techniques, such as radioimmunoassay. This technique also has advantages such as quicker results and use of less dangerous reagents. Even though a limitation of this study is the absence of paired comparison with a “gold standard” technique, due to the lack of specific tests in the country, our results are comparable to those reported by previous studies. Further comparative analyses with a larger horse population and specific grouping by breed should be done in the future.
Previous studies have shown some TT4 decrease during moderate or severe disease; however, FT4 concentrations are not affected. For this reason, FT4 may be a good indicator of true thyroid gland status in ill horses (Hilderbran et al. 2014).
Our results are also relevant because they include healthy, non-stressed horses of several breeds under tropical conditions. Costa Rica is located between the Equator and the Tropic of Cancer, resulting in quite stable tropical weather conditions throughout the year, with two seasons determined by different patterns of precipitation, known as rainy and dry season (MINAET & IMN 2009).
Even though the daylight length during the study period was fairly constant, with a maximum variation of 55 minutes (IMN 2015), an effect was observed on TT4 levels, since as the daylight length increased the TT4 values were lower. Under tropical conditions, an increase in daylight length is usually associated with warmer conditions, which might lead to a slight decrease on the metabolic rate, although the mean weather temperature, which varied only 10.2°C during the study period, did not have a significant effect on TT4 or FT4. On the other hand, some authors have also described that longer photoperiod associated with seasonal changes usually results in an increased conversion of thyroid hormones to the metabolically active form of tri-iodothyronine (Wood & Loudon 2014).
The geographical location of Costa Rica within the so-called Pacific Ring of Fire provides a mountainous and volcanic geography, with an altitude ranging from sea level up to 3800 meters above sea level (masl). Since altitude modifies air density, air temperature and pressure are influenced, leading to warmer conditions closer to sea level (MINAET & IMN 2009). In this study temperature conditions influenced basal cortisol levels, resulting in lower values under warmer conditions, which were associated with lower altitudes and seasonal changes. Cortisol levels are associated with the circadian rhythm under normal conditions (Cordero et al. 2012) and with stress levels in most animals and humans, which in this case might be associated with cooler conditions.
Another interesting finding was that FT4 levels increased with precipitation. This finding was not linked to temperature changes, since no significant relationship was found; however, it could be related to changes in air pressure or humidity associated with precipitation, which might affect heat loss and thyroidal regulation (Mullur et al. 2014).
Basal cortisol concentration in horses is susceptible to increases induced by manipulation stress, circadian rhythm, exercise, transportation, sexual excitement, restraint via twitch, hypoglycemia and isolation stress (Haritou et al. 2008; Peeters et al. 2011; Ayala et al. 2012). For these reasons, establishing a reference interval for basal cortisol is difficult. However, minimal handling of horses allowed us to confirm that the Tosoh® AIA-360 analyzer is useful to measure this hormone showing results expected based on international reference literature.
With this information, further ACTH stimulation and dexamethasone suppression tests can be used in order to diagnose adrenocortical disorders. The usefulness of the AIA-360® for dexamethasone inhibition test has already been shown in dogs (Suárez-Esquivel & Castro-Ramírez 2012; Higgs et al. 2014). Based on the close similarity of steroids in mammals (Lathe & Kotelevtsev 2014) and the good results obtained through basal cortisol measurements in our study, we expect this technique will also be suitable for detection of adrenal conditions in horses and monitoring of their stress, welfare, competition performance and as a prognostic tool in colic (Mair et al. 2014).
Finally, this is the first study in which thyroid and cortisol values are reported for Costa Rican horses and the first report of the use of AIA-360® for horses’ serum analysis.
Conclusions
AIA-360® is useful and reliable for thyroid hormone and cortisol measurement in horses. A reference interval for TT4 and FT4 is available for thyroid evaluation in Costa Rican horses using the AIA-360®.
Acknowledgments
We would like to thank José Huwiler, Marisa Fernández and Javier Coen for providing the samples, the personnel from the Clinical Analysis Laboratory (Laboratorio de Análisis Clínicos) from the School of Veterinary Medicine, Universidad Nacional, for their technical and logistical assistance, and Bernardo Vargas for the statistical analysis. This project was sponsored by Fondo Institucional de Desarrollo Académico de la Universidad Nacional (FIDA-UNA-2012).
References
Ayala, I., N.F. Martos, G. Silvan, C. Gutierrez-Panizo, J.G. Clavel & Illera J.C. 2012. Cortisol, adrenocorticotropic hormone, serotonin, adrenaline and noradrenaline serum concentrations in relation to disease and stress in the horse. Res. Vet. Sci. 93:103–107. doi:10.1016/j.rvsc.2011.05.013.
Breuhaus, B.A., K.R. Refsal & Beyerlein S.L. 2006. Measurement of free thyroxine concentration in horses by equilibrium dialysis. J. Vet. Intern. Med. 20:371–376. doi:10.1892/0891-6640(2006)20[371:MOFTCI]2.0.CO;2.
Cordero, M., B.W. Brorsen & McFarlane D. 2012. Circadian and circannual rhythms of cortisol, ACTH, and α-melanocyte-stimulating hormone in healthy horses. Domest. Anim. Endocrinol. 43:317–324. doi:10.1016/j.domaniend.2012.05.005.
Di Rienzo, J.A., F. Casanove, M.G. Balzarini, L. Gonzalez, M. Tablada & Robledo C.W. 2014. InfoStat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba. http://www.infostat.com.ar.
Dybdal, N., K. Hargreaves, J. Madigan, D. Gribble, P. Kennedy & Stabenfeldt G. 1994. Diagnostic testing for pituitary pars intermedia dysfunction in horses. J. Am. Vet. Med. Assoc. 204:627–32.
Eshratkhah, B., H. Rajabian, D. Namvar, S. Eshratkhah & Mohammadi Bastam S. 2010. Comparative study on determination of plasma thyroid hormones by chemiluminescence and electrochemiluminescence immunoassay methods in sheep. Comp. Clin. Path. 20:135–138. doi:10.1007/s00580-010-0967-8.
Graves, E. A., H.C. Schott, J.V. Marteniuk, K.R. Refsal & Nachreiner R.F. 2006. Thyroid hormone responses to endurance exercise. Equine Vet. J. Suppl. 36:32–36. doi:10.1111/j.2042-3306.2006.tb05509.x.
Haritou, S.J., A.R. Zylstra, C. Ralli, S. Turner & Tortonese D.J. 2008. Seasonal changes in circadian peripheral plasma concentrations of melatonin, serotonin, dopamine and cortisol in aged horses with Cushing’s disease under natural photoperiod. J. Neuroendocrinol. 20:988–996. doi:10.1111/j.1365-2826.2008.01751.x.
Higgs, P., M. Costa, A. Freke & Papasouliotis K. 2014. Measurement of thyroxine and cortisol in canine and feline blood samples using two immunoassay analysers. J. Small Anim. Pract. 55:153–159. doi:10.1111/jsap.12181.
Hilderbran, A.C., B.A. Breuhaus & Refsal K.R. 2014. Nonthyroidal illness syndrome in adult horses. J. Vet. Intern. Med. 28:609–617. doi:10.1111/jvim.12274.
IMN (Instituto Meteorológico Nacional de Costa Rica). 2015. Datos e información meteorológica de Costa Rica en el periodo de febrero a setiembre de 2014. San José, Costa Rica. DI-153-0615/ DI-179-0615 pp.
Kheirkhah, H.A. & Hassanpour A. 2013. Evaluation of serum levels of thyroid hormones and cortisol in arabian horses with gastric ulcer. 2:109–115.
Lathe, R. & Kotelevtsev Y. 2014. Steroid signaling: Ligand-binding promiscuity, molecular symmetry, and the need for gating. Steroids. 82:14–22. doi:10.1016/j.steroids.2014.01.002.
Liburt, N.R., K.H. McKeever, K. Malinowski, D.N. Smarsh & Avenatti R. 2013. Response of the hypothalamic-pituitary-adrenal axis and glucose homeostasis during and in recovery from acute exercise, before and after training in old and young Standardbred mares. J. Equine Vet. Sci. 33:327–328. doi:10.1016/j.jevs.2013.03.024.
Mair, T. & Sherlock C. 2009. Daily serum concentrations in horses with colic requiring exploratory celiotomy. In 55th Annual Convention of the American Association of Equine Practitioners. 483–486.
Mair, T.S., C.E. Sherlock & Boden L.A. 2014. Serum cortisol concentrations in horses with colic. Vet. J. 201:370–377. doi:10.1016/j.tvjl.2014.06.005.
Medica, P., E. Fazio, C. Cravana & Ferlazzo A. 2011. Influence of endemic goitre areas on thyroid hormones in horses. Animal. 5:82–87. doi:10.1017/S175173111000145X.
Mendoza, F.J., R.A. Perez-Ecija, R.E. Toribio & Estepa J.C. 2013. Thyroid hormone concentrations differ between donkeys and horses. Equine Vet. J. 45:214–218. doi:10.1111/j.2042-3306.2012.00622.x.
Meredith, T.B. & Dobrinski I. 2004. Thyroid function and pregnancy status in broodmares. J. Am. Vet. Med. Assoc. 224:892–4.
MINAET (Ministerio de Ambiente, Energía y Telecomunicaciones) & IMN (Instituto Meteorológico Nacional de Costa Rica). 2009. Segunda Comunicación Nacional a la Convención Marco de la Naciones Unidas sobre Cambio Climático. San José Costa Rica. 67-78 pp.
Morris, D.D. & García M.C. 1985. Phenylbutazone and anabolic steroids on adrenal and thyroid gland function test in healthy horses. Am. J. Vet. Res. 46:359–364.
Mullur, R., Y.Y. Liu & Brent G.A. 2014. Thyroid hormone regulation of metabolism. Physiol. Rev. 94:355–382. doi:10.1152/physrev.00030.2013.
Peeters, M., J. Sulon, J.F. Beckers, D. Ledoux & Vandenheede M. 2011. Comparison between blood serum and salivary cortisol concentrations in horses using an adrenocorticotropic hormone challenge. Equine Vet. J. 43:487–493. doi:10.1111/j.2042-3306.2010.00294.x.
Schenk, P., R. Nachreiner, K. Refsal, M. Mazaki-Tovi & Sist M. 2012. Endocrinology reference ranges: Diagnostic Center for Pupulation and Animal Healt- Michigan State University. WEBCD.ENDO.REF.004.0 9. 1.
Schott, H.C. 2002. Pituitary pars intermedia dysfunction: equine Cushing’s disease. Vet. Clin. North Am. Equine Pract. 18:237–70.
Singh, A.K., Y. Jiang, T. White & Spassova D. 1997. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J. Vet. Diagn. Invest. 9:261–268. doi:10.1177/104063879700900307.
Suárez-Esquivel, M. & Castro-Ramírez L. 2012. Niveles séricos de tetrayodotironina, triyodotironina y cortisol en caninos de Costa Rica mediante un analizador de inmunoensayo. Rev. Ciencias Vet. 30:25–37.
Wheeler, M.J. 2013. A Short History of Hormone Meassurement. Methods Mol. Biol. 1065:1–6. doi:10.1007/978-1-62703-616-0_1.
Wood, S. & Loudon A. 2014. Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J. Endocrinol. 222:R39–R59. doi:10.1530/JOE-14-0141.

Artículo por Revista Ciencias Veterinarias se distribuye bajo una Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Costa Rica License.
Basada en una obra en http://www.revistas.una.ac.cr/index.php/veterinaria/index.
Permisos que vayan más allá de lo cubierto por esta licencia pueden encontrarse en ciencias.veterinarias.cr@una.cr.